Gene therapies are promising treatment options for diseases that are considered untreatable. These therapies are designed by either of three methods, gene silencing, gene replacement, and gene editing to achieve the final therapy outcome. Although there are various methods to transfer the therapeutic gene into the target cells, viral vectors such as adeno-associated viruses (AAVs) seem to be the most preferred ones.
Gene therapy is a powerful therapeutic approach which relies on the modification or over-expression or suppression of gene expression. The conventional therapeutic approaches like use of small molecule inhibitors and biologics can't provide mitigation of genetic anomalies. Inherited genetic disorders involving mutation or deletion of genes may lead to defective metabolism, cell cycle, receptor-ligand interactions, protein synthesis and others. Hence, the unmet medical need in the area of genetic abnormalities is fulfilled by gene therapy. The past decade has seen great interest in the area of gene therapy with the approval of gene therapy based medicine Onpattro in 2018. In this editorial we provide an overview of the widely used methods for gene transfer, challenges in gene therapy and the applications of gene therapy in medicine.
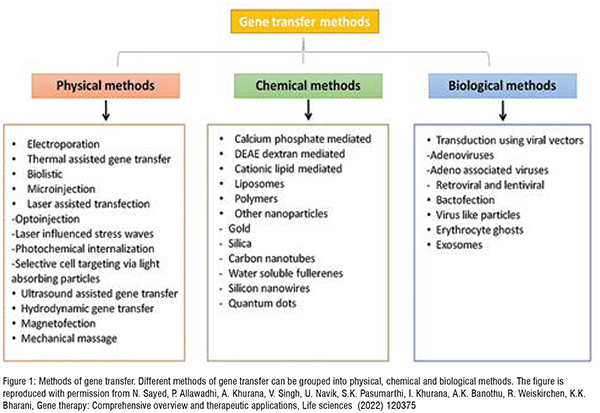
Gene therapy relies on the process of transferring engineered genes to the targeted cells. Gene transfer is a process that aims to induce the synthesis of desired protein by inserting copies of manipulated genes into the living cells. Various methods are employed to transfer the genes into the target cells, which can be listed as physical methods, chemical methods, and biological methods. Several factors influence the choice of employing one of these methods for gene transfer, which include ease of use, efficiency, cost, reproducibility, toxicity, and mechanism of delivery. Figure 1 summarises the commonly used methods of gene transfer.
Physical methods help genes to enter the cell by exerting physical force on the cell membrane, thereby increasing the permeability of the cell. This method carries a risk of injury to cells due to the sheer physical force applied to it.
Physical gene transfer methods include electroporation, biolistic, microinjection, laser, elevated temperature, ultrasound, and hydrodynamic applications.
Chemical methods help the genetic material to enter the cell by forming complexes. The chemical agents used in this method include calcium phosphate and diethylaminoethyl (DEAE)-dextran. These chemical compounds are mixed with DNA to form fine co-precipitates (complexes) which enter the cell through phagocytosis. Although this method is comparatively inexpensive, low transfection efficiency is considered a major disadvantage.
Biological methods used for gene transfer employ either viral transfection vector carriers or non-viral transfection vector carriers. Viral vectors can be adeno-associated viruses or lentivirus, whereas non-viral vectors include lipoplex and polyplex. Adenoviruses and lentivirus work differently, for instance, adenoviruses do not integrate within the host cell genome and have an episomal expression, whereas lentiviruses integrate inside the host genome which results in the formation of a stable and permanent expression of the gene and its products. Although biological methods are highly effective there remain noticeable limitations such as large-scale virus production, immunogenicity, toxicity, and insertion of mutagenesis (in the case of integrating viruses).
In addition to the three methods discussed, there are novel delivery systems for gene transfer, which include cationic lipid-mediated transfection, liposomes, polymers, virus-like particles, erythrocyte ghost, exosomes, and other nanoparticles. Although some of these methods are considered better compared to the chemical methods in terms of efficiency, they cannot replace viral vectors. For instance, the transfection systems based on cationic lipid molecules are simple to synthesise and reliable but show disadvantages such as heterogeneity and structural instability, toxicity, and inactivation in blood with a lack of targeted delivery. Similarly, liposomes are also better than chemical methods but are not relatively efficacious as viral vectors.
Major barriers to gene delivery include barriers to ease of formulation and largescale production of vectors coupled with the stability and storage of these products. In addition to these, there are three main barriers such as degradation of free plasmid DNA in the circulation, targeted delivery and intracellular trafficking, and efficient processing of gene delivery systems within the cell.
Free plasmid DNA is vulnerable to degradation within the circulation, cationic lips such as DOTAP (1,2-dioleoyl-3-trimethylammonium propane) and DOPE (dio-leoylphosphatidylethanolamine) are employed to prevent DNA degradation. These cationic lips are used to encapsulate the plasmid and protect it from the enzymatic environment outside the cell.
Once the plasmid DNAs are protected from the circulatory environment, the next phase is to protect them from the extracellular matrix surrounding the cell, as this environment limits the direct delivery of macromolecules inside the target cell. One of the main barriers to successful gene delivery is the release of DNA from the endosome before its degradation at the lysosomal level. Employing liposomes, cationic polymers polyethylenimine (PEI), and fusogenic lipids (such as DOPE, and cholesterol) are helpful for endosomal escape. For instance, Lipoplexes are employed to help the DNA escape from endosome through the fusion of liposome with the endosomal membrane.
Although the DNA is protected from circulation and extracellular matrix, there remains a challenge to transferring the genes from exogenous genetic material from the cell membrane to the nucleus. Most of the methods for enhancing nuclear import include exploitation of the cellular protein nuclear import machinery.
Gene therapies have the potential to treat many disease conditions such as cancer, cardiovascular disorders, infectious disease, and inherited disorders, among others. The success factors for gene therapy can be two-fold, being less prone to face problems of resistance, and being a permanent substitute for patients born with genetic disorders. Gene therapy has some serious side effects such as undesirable changes in the host body due to the risk of viral mutagenesis (when retrovirus and adenovirus are used as vectors).
Gene therapies are in clinical trials for various cancers such as lung cancer, prostate cancer, and melanoma. Currently, recombinant cancer vaccines are generated by using gene therapy. In this process, patients’ cancer cells (autologous) or already available cancer cell lines (allogenic) are harvested and allowed to grow in-vitro. Thereafter, the cells are modified by inserting one or more genes, which are mostly cytokine genes. These modified cells are further grown in-vitro, killed, and the released cellular components are assembled into a vaccine. There is a good clinical trial response using vaccine therapy for lung and prostate cancer. For instance, the GVAX vaccinereduces the time of progression of these diseases and also the tumor doubling time. Owing to the recent advancements in gene therapy this may become a major therapy area for treating cancer in the coming future.
In addition to cancer, gene therapies are effective in treating heart illnesses. Sickness correction can be achieved by infusing a normal copy of a gene in place of a defective one to achieve disease correction. Viral vectors such as AAVs are considered safe as they do not cause any disease in human beings. Infectious diseases that are not controlled by traditional clinical methods are targets for gene therapies. The gene therapies should be designed to inhibit or express the action of the target genes or gene products which can aid in the treatment. Neurodegenerative patients can achieve therapeutic benefits from gene therapies which can help in neurorestorative, neuroprotection, direct correction of pathogenic mechanism and symptom control. These applications for gene therapy are not exhaustive as the research for genes as therapeutic agents is being continued for many other conditions such as metabolic diseases.
Extensive research to develop novel gene therapies is in progress. The demand for such therapeutics is very high, due to their unique therapeutic benefit of healing the disease permanently from the genetic level. However, challenges
such as difficulty in production scaleup, and the need for high transfection efficiency with low toxicity need to be addressed to produce safe gene delivery systems. Clinical research for gene therapies for various disorders shows the promise that gene therapies can hold in the future as the understanding of critical aspects of fundamental molecular physiology and pathology are being advanced. Improvements made in gene therapy strategies will pave the way for the treatment of genetic maladies in the near future.
References:
[1] N. Sayed, P. Allawadhi, A. Khurana, V. Singh, U. Navik, S.K. Pasumarthi, I. Khurana, A.K. Banothu, R. Weiskirchen, K.K. Bharani, Gene therapy: Comprehensive overview and therapeutic applications, Life sciences (2022) 120375.
[2] A. Akinc, M.A. Maier, M. Manoharan, K. Fitzgerald, M. Jayaraman, S. Barros, S. Ansell, X. Du, M.J. Hope, T.D. Madden, The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs, Nature nanotechnology 14(12) (2019) 1084-1087.
[3] W. Xu, X. Jiang, L. Huang, RNA interference technology, Comprehensive Biotechnology (2019) 560.
[4] A.J. Mellott, M.L. Forrest, M.S. Detamore, Physical non-viral gene delivery methods for tissue engineering, Annals of biomedical engineering 41(3) (2013) 446-468.
[5] J. Sambrook, D.W. Russell, Calcium-phosphate-mediated transfection of eukaryotic cells with plasmid DNAs, Cold Spring Harbor Protocols 2006(1) (2006) pdb. prot3871.
[6] D. Bouard, N. Alazard‐Dany, F.L. Cosset, Viral vectors: from virology to transgene expression, British journal of pharmacology 157(2) (2009) 153-165.
[7] M. Ruponen, P. Honkakoski, S. Rönkkö, J. Pelkonen, M. Tammi, A. Urtti, Extracellular and intracellular barriers in non-viral gene delivery, Journal of controlled release 93(2) (2003) 213-217.