A localised metronomic approach sustaining low chemotherapy drug concentrations in the peritoneal cavity can be very effective for late stage ovarian cancer therapy. Biodegradable polymeric woven nanotextiles can serve as suturable intraperitoneal depots by providing extended drug elution for>2 months, wherein the metronomic doses render both anti-angiogenic and anti-tumour effects.
Overall death cases from Ovarian Cancer (OC) accounts to be the highest among different gynaecological malignancies affecting cervix, uterus and ovaries. The extremely poor survival rates of 15-30 per cent at late stages of OC is mainly due to the advanced-stage detection of the disease, viz, Stage III or IV, wherein, the aggressive tumour spreads within and beyond the peritoneum. The highly asymptomatic nature of the disease coupled with inadequate screening methods also contribute to the disease lethality. Epithelial Ovarian Cancer (EOC), wherein the tumour originates from the surface layer of ovaries, constitute about 75 per cent of the total disease incidences and the long term survival of EOC patients remains poor according to recent reports. Unlike other tumours, EOC metastasizes via transcoelomic route by which the malignant cells exfoliate along the peritoneal cavity by virtue of peritoneal fluid flow. Hence, peritoneum is the primary and predominant site of EOC metastasis (Stage III) which subsequently spreads to omentum and later to distal organs such as liver and lung (Stage IV). Peritoneal metastasis is mainly favoured by the absence of an anatomical barrier within peritoneum and the continuous flow of fluid rich in tumour cells. Obstruction of lymphatic vessels by tumour cells impairs the drainage mechanism, particularly in late stages, thereby leading to the accumulation of massive amounts of peritoneal fluid, a condition termed as cites. Ascitic fluid circulation increases the rate of metastatic tumour progression at distant sites beyond abdomen. Furthermore, EOC is considered to be a richly vascularised tumour which is dependent on Vascular Endothelial Growth Factor (VEGF) mediated angiogenesis, which concurrently enhances the peritoneal vascular permeability increasing the ascites accumulation.
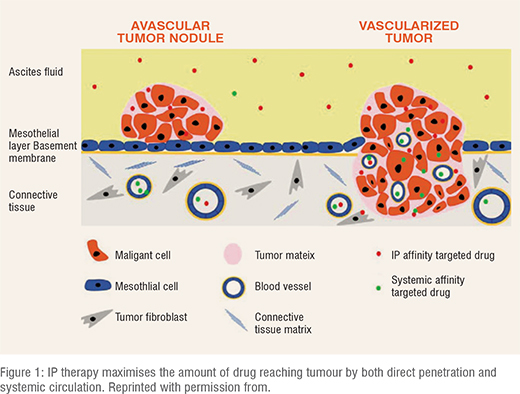
The current mainstay of ovarian cancer therapy is debulking surgery to remove peritoneal tumour nodules, followed by intravenous (IV) chemotherapy of a combination of platinum and taxane drugs at their Maximum Tolerated Doses (MTD). However, the cumulative, doselimiting, and irreversible toxicity issues caused by IV drug doses cannot be negated, although the overall therapy provides substantial survival benefits. Most importantly, almost 80-90 per cent of EOC patients manifest a disease relapse even after the conventional therapy schedule involving surgery and standard six cycles of IV chemotherapy. Acquisition of resistance owing to frequent drug administration is another phenomenon typically observed upon tumour relapse. In such a context, a localised intervention facilitating chemotherapy drug delivery exclusively to peritoneal tumour nodules would enhance drug retention within peritoneal cavity and associated organs. Such an approach termed intraperitoneal(IP) chemotherapy mitigates the systemic toxic effects imparted by IV chemotherapy. The existence of peritoneal plasma barrier and physico-chemical properties of drugs with a high peritoneum/plasma AUC ratio helps in exploiting the pharmacokinetic advantages of IP therapy. Along with the direct peritoneal drug uptake by tumours via microcirculation, systemic drug absorption also takes place in tumour tissues and the submesothelial space of peritoneum. Additionally, IP route also enhances the targeting of avascular residual tumour nodules left after debulking, thereby maximising the overall tumour targeting (Figure 1). Although several clinical trials have established significant survival benefits conferred by IP therapy in debulked OC patients, it is still underutilised and has not yet become a standard of care in the clinics. This is due to the practical difficulties imposed by indwelling intraperitoneal catheters, which are used to administer frequent and intermittent IP doses. These include leakage and port access issues, resultant bowel obstruction and IP bolus dose-imparted toxicity. Alternatively, a different approach to circumvent these concerns caused by the intermittent and frequent drug administration at MTD, is the continuous elution of drug without sequential breaks, at lower doses. Such a dosing regimen, also called as ‘metronomic therapy’ aims at shifting the target of action of the chemo drugs from tumour cells to its vasculature, correlating with the enhanced sensitivity of endothelial precursor cells to low doses of several drugs. Metronomic dosing can also help to overcome drug resistance, cause immunomodulation and improve anti-tumour effects. Over the years, extensive pre-clinical data is available, pertaining to the advantages of IP therapy via both intermittent and metronomic dosing regimens, for refractory OC therapy. Nanoparticles, microparticles, injectable hydrogels, etc. have been developed as IP depots and investigated in detail by various groups. While nanoparticulates appeared to clear quickly from the peritoneal cavity, microparticulates caused the formation of undesirable peritoneal adhesions in animal models. Injectable depots on the contrary, retained the peritoneal drug levels, however, non-homogenous drug distribution and viscosity issues curtailed their potential application as IP depots. Microdevices formulated by Cima et al, released cisplatin for a long duration of about 42 days in peritoneal cavity of mice model. Nevertheless, these devices were found to migrate to the extra-peritoneal regions, thereby triggering a probable non-uniform drug distribution in peritoneum. Although there has been considerable progress in the development of IP drug delivery systems for refractory OC, no specific formulations have been approved by FDA till date.
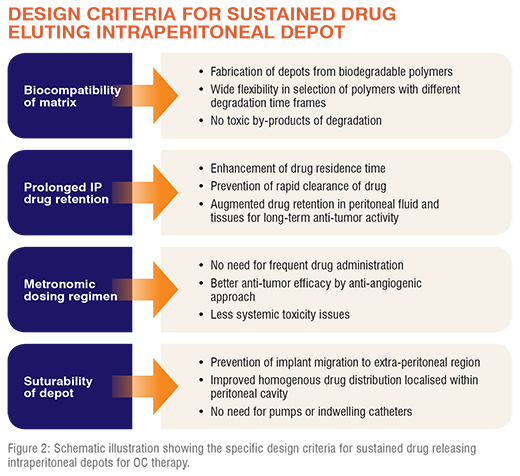
Specific design criteria needs to be considered for developing an ideal IP drug delivery platform for refractory OC therapy (Figure 2). The most important amongst these is the peritoneal drug retention that prevents rapid clearance of the drug, ensuring prolonged anti-tumour activity. This can be achieved by adopting various drug encapsulation strategies so as to improve drug residence time in the peritoneum, which improves its penetration into microscopic tumour nodules, especially post debulking surgery in EOC patients. Toxicity issues can be obviated by having precise control over the drug dosing regimen, as in metronomic scheduling of continuous low drug doses. Additionally, the depots should be fabricated from biocompatible and preferably biodegradable polymers, mainly to avoid the undesirable toxic effects which can otherwise be imparted by the matrix or its residues. Considering all these attributes, implantable depots would be ideal candidates for IP therapy owing to their ability to elute drug for prolonged durations. Moreover, considering the large peritoneal cavity space accommodating all of the abdominal organs, it is important to prevent migration of the implant to other regions. An implantable depot which can be fixed to the peritoneal wall by simple suturing can be an ideal solution. In the clinical context, this is a feasible option owing to the ease of implanting the depot after surgical debulking of tumour, ruling out the need for another surgical invasive procedure. Such a depot could facilitate a long-term drug release into peritoneal cavity and consequently prevent the chances of a probable disease relapse.
The technique of electrospinning has been established as a feasible method for the fabrication of fibrous matrices using biodegradable polymers entrapping different therapeutics and bioactive constituents. It offers wide flexibility in the selection of polymers with varied degradation time-frames and thus enables the modulation of drug release profiles. Moreover, it also aids in the fabrication of scalable quantity of continuous fibres with nano/micro morphology and different architectures, and facilitate high drug loading via appropriate choice of polymer: drug: solvent combinations. Adopting the technique of modified electrospinning using a rotating collector (our patented technology), our group has developed uniform and continuous nanofibrous yarns from the biodegradable polymer polydioxanone (PDS), entrapping the chemodrug, paclitaxel (PTX) with an efficiency of ~90 per cent. These yarns with an average diameter of ~240 µm and good mechanical strength were subsequently woven into nanotextile fabrics by applying textile technology-principles of plain weaving. Woven fabrics of different packing densities were developed by modulating weaving parameters such as number of warp/weft yarns and the distance between heddles in weaving-loom, thereby forming tightly-packed and loosely-packed implants. PTX-loaded yarns were found to render a drug release profile for over 90 days in vitro in PBS, which almost correlated with its complete degradation in vitro within 120 days. Mechanistic modelling considering the nanofibrous architecture of these yarns proved that the overall biphasic release profile was rendered by a combination of both PTX-diffusion and PDS-degradation. Drug release studies from woven fabrics showed that tightly-packed fabrics eluted PTX at a much slower rate in comparison to loosely-packed ones that released drug at a rate similar to yarns. Highly-packed matrices in general, take longer time to undergo complete hydrolytic degradation, which consequently slows down the drug release rate. Similar findings were observed for drug release experiments conducted in patient-derived peritoneal fluid samples, wherein the overall amount of drug released was higher owing to accelerated degradation of nano-fibers. Loosely-packed woven fabric implant was chosen for in vivo experiments considering its ability to offer higher loading dose and optimal drug release kinetics. In vivo experiments performed in healthy BALB/c mice by suturing the implant to peritoneal wall showed that the implants remained intact at the sutured site without any adverse effects for a duration of >56 days.
The in vivo drug release profile from the woven implant was biphasic, constituted of a burst phase within 5 days, followed by a continuous metronomic phase for nearly 60 days. This burst phase mimics the clinically administered bolus IP dose of chemotherapy drug and the metronomic regimen correlates with the maintenance therapy that would prevent disease relapse. This long-term drug release profile was also found to retain good therapeutic PTX-levels in both peritoneal fluid and all peritoneal tissues for >28 and 56 days respectively. These results were in total contrast to mice injected intraperitoneally with PTX in solution (single bolus dose as clinical control @20mg/kg dose relative to the single implantation of fabric), wherein the drug levels were found in tissues only on the first day of drug release, owing to its abrupt clearance.
IP implantation of biodegradable fabrics in ID8-VEGF OC model
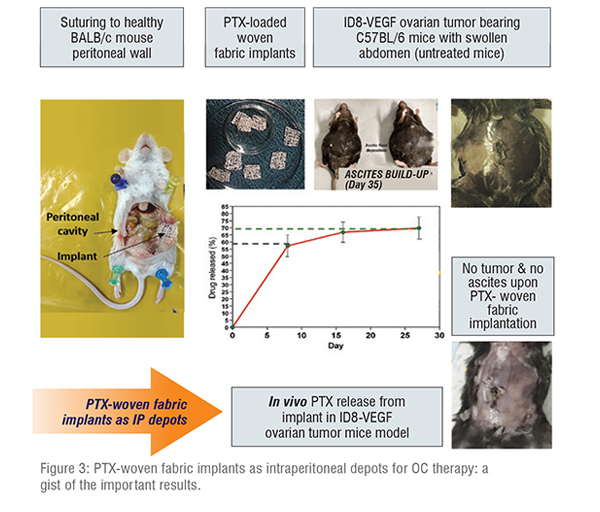
In order to study the anti-tumour effects of woven fabric implant, efficacy studies were performed in ID8-VEGF syngeneic ovarian cancer mice model developed by injecting 4 million ID8-VEGF cells intraperitoneally into C57BL/6 mice. This model was chosen owing to its physiologic resemblance of human EOC metastasis. Previous studies had confirmed the onset of tumour growth in these mice at day 8 post tumour injection, through increased VEGF levels (~36 fold higher than that of naïve mice) and presence of Tumour Associated Macrophages (TAM) in peritoneal lavage by ELISA and FACS respectively. Considering these results, PTX treatment via woven fabric implantation and IP injection were also performed on day 8 post tumour inoculation. The in vivo PTX release profile was similar to that observed in naïve mice wherein over 55 per cent and 70 per cent of total entrapped PTX were released within 7 and 28 days respectively (Figure 3).
In vivo efficacy studies
The in vivo efficacy response to PTX therapy (given at a clinical dose of 20mg/kg) was studied in ID8-VEGF tumour model over a course of 35 days post tumour injection. It was observed that control animals (untreated mice and those implanted with woven fabrics without PTX) showed a gradual development of ID8 tumour manifesting swollen abdomen with tumour nodules in peritoneal tissues and diaphragm, accompanied by a progressive accumulation of ascitic fluid (~5-7ml) by day 35. Although PTX-solution injected animals did not show tumour nodules, and only blood traces in the initial stages, metastatic lesions were present by day 24 along with ascites formation(~2ml) by day 35 (Figure 3). The reported short half-life of PTX in solution form might have hampered its peritoneal retention concurrently leading to tumour recurrence post its clearance. On the contrary, PTX-loaded woven fabric implanted animals manifested clean peritoneum consistently throughout the study, with no tumour nodules, blood traces or ascites accumulation at all time-points, resembling naïve animals. VEGF being the important regulator of angiogenesis, triggering ascitic build-up and hence tumour progression, peritoneal lavage samples retrieved from mice of all groups and different time points were assessed by ELISA. While the control groups displayed a time dependent VEGF-level increase by day 35, PTX-woven fabric implanted animals showed a gradual decrease in VEGF levels, such that its day 35 levels were ~600-fold lesser than that of control groups (also similar to naive mice).This emphasized the effect of metronomic-PTX dosing on the long-term suppression of VEGF-mediated angiogenesis and thereby tumour spread. These results of metastatic spread and angiogenesis were also confirmed by histology and immunohistochemistry using anti-CD-31 antibody respectively.
In vivo safety studies
In vivo safety attributes of woven fabric implant at day 35 were studied by analysing the serum and lavage levels of liver enzymes, Alanine aminotransferase(ALT) and Aspartate Aminotransferase(AST) as well as complete Blood Cell Count (CBC). PTX treatment was observed to bring down the elevated ALT and AST levels of control groups both in serum and lavage. A slightly higher enzyme level was noted for PTX-solution injected animals relative to woven fabric implanted ones, possibly due to the hematologic toxicity imparted by PTX bolus doses. Reduction in WBC and RBC counts noted for control animals were negated by PTX treatment, particularly for PTX-fabric implanted mice, wherein the levels were almost similar to those of naïve ones. Furthermore, neutropenia exhibited by control groups and PTX-injected animals was also absent in the case of woven fabric, wherein the levels correlated with naïve animals. These results affirmed the non-toxic effects of metronomic-PTX dosing from woven fabric implants. The bolus dosing as well as the presence of Cremophor® in PTX-solution can be considered as the reasons for toxicity imparted by PTX-solution. On the other hand, PTX-woven implants being fabricated from biocompatible and biodegradable PDS yarns did not induce any inflammatory response or other adverse events, signifying its safety profile.
Conclusions
The pharmacokinetic advantages of intraperitoneal therapy when coupled with the anti-angiogenic effects of metronomic dosing can be effectively exploited for the treatment of refractory ovarian cancer. Sustained and metronomic release of chemo drugs exclusively into the peritoneal cavity can aid in the retention of prolonged drug levels with peritoneum, thereby hampering the chances of a probable disease relapse. Nanotextile fabric implants woven from biodegradable polydioxanone yarns can serve as suturable intraperitoneal drug depots for OC therapy. These implants when sutured to the peritoneal wall of ovarian tumour bearing animals were found to render substantial anti-tumour effects with enhanced safety for a long period owing to the continuous elution of metronomic PTX upon single implantation. This low dose approach also aided in the mitigation of the adverse toxic effects of bolus PTX-doses. The overall strategy is also a clinically translatable technology which can be practically adopted by clinicians in a patient-compliant manner.
Acknowledgements
Authors acknowledge financial support obtained from Pilot Project Grants, Program for Young Investigators in Cancer Biology of the Department of Biotechnology, Government of India and United States National Cancer Institute of the National Institute of Health through grants R21-CA179652 and R56- CA198492, and the Northeastern University-Dana Farber Cancer Center Joint Program on Cancer Drug Development. SP acknowledges DST-INSPIRE, Government of India for her Senior Research Fellowship and Amrita Vishwa Vidyapeetham for PhD Scholar’s Fellowship and other infrastructural support.