Many new approaches have been considered for large-scale chromatography including membrane adsorbers. The benefits and drawbacks of these devices and their potential impact on the biopharmaceutical industry are discussed.
Upstream productivity in the biomanufacturing industry is not being matched by improvements downstream. For processes such as packed-bed chromatography that cannot be scaled up indefinitely, the future is starting to look bleak. Manufacturers around the world are approaching the limits of productivity and scalability, and innovative downstream processing (DSP) solutions are required to address this challenging issue. Many new approaches have been considered including the re-evaluation of simpler separation technologies and the development of high-technology solutions such as membrane adsorbers.
In the biopharmaceutical industry, downstream processing is completely dependent on chromatography. Different resins exploit different principles, so that the purification of specific proteins, such as antibodies, from cell culture broth is achieved by using two, three or even more types of chromatography in series. There are three typical steps in the purification of antibodies: one based on Protein A affinity chromatography for initial product capture, followed by two steps of polishing. In many cases, one is based on Anion Exchange (AEX) operating in flow-through mode to retain negatively-charged impurities such as Host Cell Protein (HCP), host cell DNA / RNA, leached Protein A and endotoxins, and the other is based on either Cation Exchange (CEX) or hydrophobic interactions to resolve the intact dimeric antibody from aggregates and degradation products.
Although packed-bed chromatography is a successful technology that dominates the biopharmaceutical industry, all is not well in the world of antibody manufacturing. There is all-too-frequent talk of bottlenecks in DSP. The success of antibody-based products coupled with pressure on manufacturers to increase their yields while reducing the cost of goods has driven them to focus almost exclusively on the upstream phase of production. Therefore, 5 g/L titres can now be achieved routinely in mammalian cells, representing a 100-fold increase over the last two decades. At the same time, upstream manufacturing capacities have increased dramatically, with many manufacturers choosing to operate several 10,000-L bioreactors simultaneously. In contrast, little attention has been paid to DSP, and the same standard technologies that were developed to handle titres of 50-100 mg/L are still in use today.
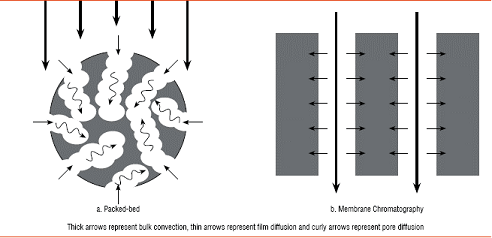
Figure 1: Principal solute transport mechanisms
The bottleneck in process-scale chromatography negates any advantages of scaling up earlier process units, since bind-and-elute chromatography steps are driven by mass rather than volume. This means that savings made upstream do not translate into increased productivity during purification. Larger columns also impact directly on facility layouts, costs and infrastructure because the space and buffer volumes for all steps also increase. As a consequence, pool and buffer volumes act as serious limitations when it comes to the introduction of high-titre processes into existing facilities.
There are also operational problems with columns operating in flow-through mode. In resin-based AEX media, the transport of solutes to their binding sites relies on pore diffusion, but the contaminants are often large molecules-DNA and viruses-which do not readily diffuse into the pores. This causes mass transfer resistance and lowers the column efficiency because large molecules can only bind to the outer surface of the bead and longer residence time is required to find binding ligands inside the resin particles. The only solutions are to use a greater column bed height and / or to reduce the linear flow rate, both of which will have an impact on the overall productivity. Therefore, to keep up with the process demand, most traditional polishing steps operate at a flow rate of between 100 and 150 cm/h and use significantly oversized columns to accommodate this.
Apart from productivity and economic issues, large columns also suffer from scale-related packing problems such as hysteresis, edge-effects and resin compression, which result in unpredictable fluid distribution and pressure drops.
The shift in perspective from upstream to downstream processing has become more evident in the last few years as experts have begun to acknowledge that improved productivity cannot rely on more efficient upstream production alone, and that downstream improvements must consider the entire production train and not just single operations to avoid shifting of the bottleneck from one operation to another. Various alternatives have been put forward either to replace column chromatography or to reduce the load of impurities in the feedstream so that one or more chromatography steps can be eliminated. Many of these employ low-technology methods that are inexpensive but are applicable on a large scale. For example, techniques such as flocculation, precipitation and multiphase extraction can be used in combination with conventional cell separation techniques such as centrifugation and microfiltration to remove residual particulates and soluble impurities that might otherwise increase the burden on downstream polishing steps.
Some engineering-based solutions have also been implemented, including simulated moving bed chromatography, which allows higher throughput without increasing buffer consumption. High-tech solutions to the capacity crunch in downstream processing offer innovative replacements for traditional packed-bed chromatography. Examples include monoliths, charged ultrafiltration membranes and membrane adsorbers, all of which carry out analogous functions at reduced cost.
Membranes are already integral to many bioprocesses because disposable filters have proven to be inexpensive and convenient while matching the performance of their fixed, stainless steel counterparts. For many unit operations, particularly filtration and media / buffer storage, disposable devices are now so commonly used that they are regarded as a normal practice. The industry is keenly aware of the advantages they provide in terms of reduced down-time, flexibility in process development / scaling, and the elimination of cleaning and validation costs, especially where multiple products are manufactured in the same facility.
Interest in membrane chromatography is growing because of the success of disposable membrane filters, yet there is a lack of appreciation of the many advantages they offer in downstream processing. Not only do they share all the economic and versatility benefits listed above for filters, but they also have specific functional advantages over equivalent packed-bed columns. These are:
Convenience - Membrane adsorbers are disposable modules. They are supplied ready to use and can be discarded once exhausted. There is no cleaning or validation, no need for life-time studies, no packing, re-packing and recycling. Fouled modules can be replaced with new ones more conveniently and cheaply than packed resins.
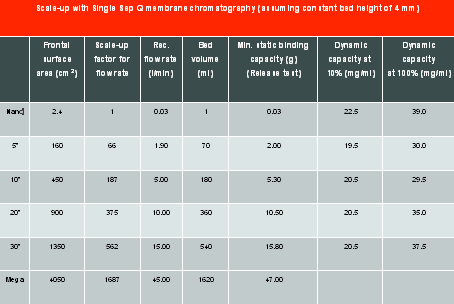
Scalability - Manufacturers offer devices of various sizes that can be plugged into existing process trains, thereby allowing required rapid scale-up or scale-down. Importantly, as shown in Table 1, there is linear scale-up for important parameters such as frontal surface area, bed volume, flow rate and static binding capacity, while normalised dynamic capacity remains fairly constant at 10 per cent or complete breakthrough.
Small footprint - Membrane adsorbers are typically available in a cartridge format wherein 10 to 15 membranes are stacked. The consequences in terms of buffer usage are impressive, reducing buffer consumption to less than 1 per cent of the amount required for an equivalent packed column. The impact of this is felt not only in the reduced buffer usage, but also in the facility requirements in terms of buffer storage, preparation time and waste management. The savings made here more than offset the cost of the modules themselves.
Operational performance - In flow-through applications such as AEX in antibody polishing, membranes outperform columns because the transport of solutes to their binding sites occurs mainly by convection, while pore diffusion is minimal (Figure 1). Because of these hydrodynamic benefits, membrane adsorbers can operate at much greater flow rates than columns, reducing buffer consumption even more and shortening the overall process time by up to 100-fold. The use of membrane adsorbers can be viewed as the equivalent of shortening traditional columns to near zero length, allowing large-scale processes to run with only a small pressure drop at very high flow rates. For example, polishing with an anion exchange membrane can be conducted with a bed height of 4 mm at flow rates of more than 600 cm/h, providing a high frontal surface area to bed height ratio. Small-volume disposable membrane chromatography devices can now handle more than 10 L per minute/bar/m2.
Virus clearance - All biomanufacturing processes involving mammalian cells must include steps to clear and inactivate viruses. The efficiency of these steps must be demonstrated via spiking studies using a range of model viruses with properties representing possible contaminants. Multiple studies have now demonstrated that membrane adsorbers are at least as efficient at virus removal as equivalent columns, and due to the hydrodynamic benefits mentioned above often exceed columns in terms of virus clearance. Log reduction values of >5 are routinely achieved for most model viruses. The performance studies corroborate the fact that packed columns and membrane chromatography devices are both capable of removing trace contaminants and clearing viruses in polishing applications. Apart from the handling concept, the main difference between the two formats is the load capacity at flow rates acceptable for large-scale manufacturing. Multi-layer Q membranes achieve much greater productivity compared to equivalent volumes of resin with no loss of performance in contaminant and virus removal. For this reason, the volume of a disposable membrane device is typically 5 per cent of that required for a conventional column, which needs to be oversized to accommodate the volumetric flow rate.
Integrity - Functionality and integrity tests demonstrate that even with bed heights of less than 1 cm, membrane stacks are robust and reliable.
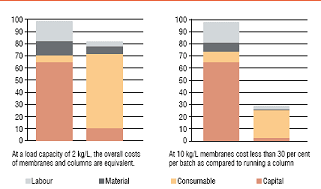
Figure 2: Comparative results from a cost model*
comparing packed-bed and membrane AEX
chromatography in flow through modeEconomy - Several cost comparisons have been carried out for membrane adsorbers and resins used for flow-through polishing of antibodies. These indicate that column chromatography is overall more economical for low loading rates whereas membranes break even with them at a load of 2 kg/L. At higher loads, membranes become more economical, which means that as the productivity of a process increases membranes become the better choice for polishing. The models were comprehensively researched and they took into account factors such as capital charges (higher for columns due to the need for capital equipment purchase), labour (higher for columns due to the time required to prepare buffer, pack and re-pack the columns, and time for cleaning and validation studies), consumables (higher for membranes because of the cost of each membrane device, but offset to an increasing extent at higher scales by the increased usage of buffer and water for injection in column chromatography) and material costs (including utilities, spare parts and the resin, which is significantly higher for columns). The outcome of the cost analysis can be seen in Figure 2.
Early membrane adsorbers suffered from problems related to both adsorptive capacity and device performance, e.g. low loading capacity, membrane fouling, leaching and suboptimal fluid distribution leading to a substantial performance loss during scale-up. Many of these issues have been addressed by the development of more suitable matrices, better device housings and improved surface chemistries. For flow-through applications where trace impurities are retained, membrane adsorbers now out-perform columns under most conditions and therefore are adopted by more and more manufacturers. Performance tests routinely show reduction of HCPs and DNA below detection levels.
Currently, the greatest limitation of membrane adsorbers is in the bind-and-elute operations for small molecules, where the binding capacity is lower than equivalent columns. Thus, packed-bed chromatography is still the preferred unit operation for capturing molecules of 200 kDa, especially when peak cutting, gradients etc. are required for the separation of closely related species. Membrane chromatography, on the other hand, is more suited to simple on-or-off binding scenarios such as polishing and the capture of large molecules from diluted feed streams. Even so, membranes are beginning to encroach on bind-and-elute operations, the traditional strong-hold of column chromatography. It is now possible to use both AEX and CEX in antibody polishing, the latter to purify the antibody from product-related impurities such as aggregates and degradation species, as well as positively-charged HCPs. Here, the first polishing step plays to the strengths of membrane chromatography-it is carried out in flow-through mode to retain large, negatively-charged contaminants such as DNA, RNA, leached Protein A, HCPs, endotoxins and viruses-whereas the second step exploits the fact that the feedstream is relatively pure at this stage, and therefore the binding capacity of the membrane is unlikely to be challenged.
There is no doubt at all that pressure on downstream processing will increase over the next decade. There are more products in the pipeline, a greater demand for drugs, more awareness of regulatory issues and greater acceptance of biotechnology, plus lots of biotechnology-derived drugs coming off patent leading to an increase in the manufacture of biogenerics. The outlook for biochromatography is therefore challenging because today's processes based on the use of oversized columns stuffed with expensive resins are becoming increasingly uncompetitive. The continual scaling up of processes is unsustainable, and needs to be given a serious thought.
More and more companies will look at adopting membrane chromatography for polishing, since flow-through operations are considerably less expensive and more convenient when membrane adsorbers are used. Any cost savings in processing can lead directly to a reduced cost of goods, and this can only be good for the industry and, ultimately, its customers. The convenience of membrane adsorbers is possibly more important than the economic benefits, since the adoption of a fully disposable process train with plastic buffer and media bags, disposable bioreactors, single-use filters and single-use adsorbers will eliminate the need for cleaning and validation. It would also help the facilities to produce multiple products with minimal turn-around times, maximising the use of resources and minimising the use of space. However, the main bottleneck in DSP will always remain at the initial capture step, since this is driven by mass rather than volume and has a knock-on effect throughout the facility.
Membrane adsorbers are unlikely to replace Protein A columns for the foreseeable future unless there is a revolutionary leap forward in the technology to allow more efficient capture. Therefore, the industry faces the prospect of further parallelisation (more columns per process) or the adoption of other technologies, e.g. innovative resins with mimetic ligands, or the replacement of Protein A with other chromatography modes that are cheaper and more scalable. If no replacement for Protein A can be found, the problem could be circumvented in a radical and unorthodox manner by simply removing the capture step all together. It is not inconceivable that processing in the future may be based almost entirely on polishing and removing contaminants from the feedstream rather than capturing the product.