Pharmaceutical manufacturers are looking to Lean Manufacturing and Six Sigma principles to help them boost operational efficiency and improve quality, while facilitating compliance.
Today, pharmaceutical manufacturers are focused, as never before, on reducing operational costs while ensuring compliance. Cost pressures are increasingly acute as many pharmaceutical manufacturers see a dwindling product pipeline, as well as greater competition from generics. To ensure that their bottom lines remain solid, pharmaceutical manufacturers are looking to increase the efficiency of their operational and manufacturing processes—optimising resources, improving efficiency, reducing waste and controlling inventory. The regulatory climate is, at last, conducive to focusing on such changes, as the U.S. FDA as well as other regulatory bodies now support approaches that seek to reduce risk by building quality into the manufacturing process from the start, as opposed to relying on end-process testing. In this climate, pharma manufacturers are looking to Lean Manufacturing and Six Sigma principles—proven in other industries—to help them boost operational efficiency and improve quality, while facilitating compliance.
Despite the pharmaceutical industry’s focus on quality, it has failed to keep up with other industries in terms of manufacturing efficiency and productivity, largely because of the cost and burden involved in revalidating any process changed in the spirit of improvement. Once manufacturers confirm or validate their processes as compliant, they traditionally have been very reticent to change them. The simple fact is that pharmaceutical manufacturers, which historically have enjoyed consistently robust profit margins, have had little economic incentive to introduce change.
The industry’s focus on maintaining the status quo in its manufacturing environment has produced inefficiency and waste. It is estimated that the potential world-wide cost savings from efficiency improvement could be as high as US$ 90 billion. While Research and Development (R&D) is generally considered a major cost centre for the pharmaceutical industry, manufacturing quietly accounts for more than twice the expense of R&D—representing, on average, 36 percent of a pharmaceutical manufacturer’s costs. The true cost of manufacturing becomes even more apparent when one considers the amount attributed to non-value-added activities and waste which is 80 percent and 50 percent, respectively.
Quality has also suffered under the status quo. It is interesting to consider that the number of drug recalls has risen sharply in recent years—three-quarters of which are attributed to manufacturing defects. The reject percentage in the pharmaceutical industry ranges from 5 percent to 10 percent (<2>
Several important factors have converged in recent years to jumpstart substantial change in how pharmaceutical manufacturers approach and manage their manufacturing operations. First, many manufacturers face a declining development pipeline as well as shrinking profit margins as they face increased competition from generic drug manufacturers. As such, they see a growing need to abandon the status quo to focus on improving productivity, efficiency and quality. At the same time, the U.S. FDA and other regulatory bodies are acknowledging that the industry has fallen behind other sectors in terms of efficiency and quality, and have begun to endorse a “quality by design model” that contrasts with the industry’s historical “quality by test” results approach. As part of this shift, the U.S. FDA launched its Process Analytical Technology (PAT) initiative, a risk-based guidance model that seeks to direct pharmaceutical manufacturers toward consistent and predictable quality (higher sigmas). The PAT approach is to build in quality improvements on the factory floor through a deep understanding of how variable process attributes affect product quality at a fundamental level.
As pharmaceutical manufacturers seek to transform manufacturing operations and enhance quality, many are turning to two highly regarded management approaches—Lean Manufacturing and Six Sigma—that have proven effective in other industries, such as electronics and auto manufacturing.
Lean Manufacturing focuses on eliminating manufacturing waste, with the objective of making manufacturers more responsive to customer demand and market changes. Six Sigma is a business process methodology that focuses on minimising variation—in product and process—to reduce product defects.
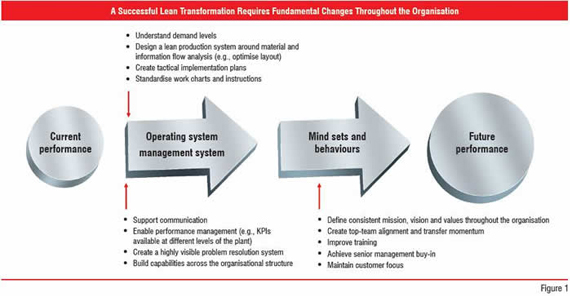
Using the methodology, one standard deviation from the mean is one sigma; therefore, manufacturers operating at ±6 sigma are operating at 99.9997 percent compliance. When pharmaceutical manufacturers implement Lean and Six Sigma concepts they have a powerful methodology to help them improve quality, compliance, productivity, costs and speed—ultimately enabling them to bring better products to market, faster and more cost effectively. To achieve transformation to a Lean Six Sigma environment, organisations must focus on change at three levels—operating systems, management systems, and mindsets / behaviours. (Figure 1) At the operating system level, manufacturers must understand demand levels, design lean production systems around material and information flow analysis (e.g., optimise layout), create tactical implementation plans, and develop standardised work charts and instructions.
When transitioning to a Lean Six Sigma environment, pharmaceutical companies must also assess their management systems at several different levels and direct changes that support Lean concepts. Transition teams must consider what management tools, including IT systems and communication tools, the company requires. The organisation must also consider how it will define or measure success, by setting Key Performance Indicators (KPIs) at different levels of production. It is also essential to create a highly visible problem resolution system to drive and institutionalise change. Finally, organisations must implement and scale these capabilities so that they span the entire value chain—which is often dizzyingly complex and can extend beyond traditional organisational boundaries. The final arena for hange—transforming mindsets and behaviours—is often the most challenging for many organisations. Individuals often fear, and in turn, resist change. This truism is especially applicable in the pharmaceutical manufacturing industry, which has, until recently, thrived despite its focus on maintaining the status quo.
In transitioning to a Lean Six Sigma environment, it is critical that the management team defines and communicates a consistent mission, vision and value system throughout organisation—always maintaining the customer focus. The transformation team should focus on achieving top-down buy-in by aligning resources to help build and transfer momentum across the organisation. Training cannot be overlooked. Transformation team must develop a comprehensive strategy that provides training opportunities at multiple points in the transformation as a means of achieving h4er organisational buy-in and competence from both management and the ranks.
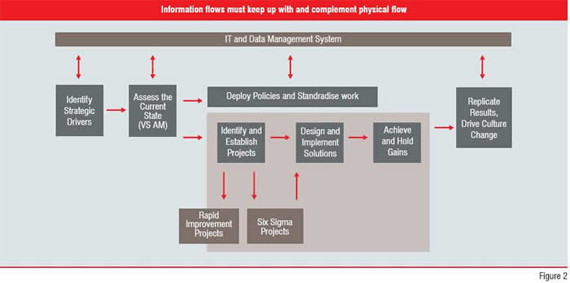
IT factors heavily in the transition to a Lean Six Sigma enterprise and the subsequent journey of continuous improvement. Because of the complexity of the pharmaceutical manufacturing environment, organisations require flexible and interoperable IT systems that provide information, not just data, across the enterprise. In a Lean Six Sigma environment, information flows must complement and keep pace with physical flows to deliver the information needed (Figure 2).
The ability to mine data and interpret it efficiently, quickly and seamlessly is also very important. Using the data and turning it into information quickly enables pharmaceutical manufacturers to outperform their competition. Having the right data readily available when it’s needed also makes it easier to respond to FDA inquiries.
IT supports several tenets essential to the implementation of a Lean Six Sigma environment, including:
Organisations require good data to make wise decisions. Most pharmaceutical manufacturers have IT environments with solutions from multiple IT vendors. These systems are often siloed, precluding the exchange of information. In addition, manufacturers often have multiple instances of applications across their various production facilities. For example, it is not unusual for pharmaceutical manufacturers to have separate data files for products and customers in different IT systems at different sites. This approach also precludes a comprehensive view of the enterprise, which is essential to a quality-by-design focus.
To obtain the end-to-end visibility needed in a Lean Six Sigma environment, pharma manufacturers must have an integration strategy for linking heterogeneous systems, creating a single source of trusted information that provides a complete picture of the operations.
A Lean Sigma Six environment requires complete confidence in the integrity of an enterprise’s supply chain, manufacturing and distribution-related data. As such, a single source of information is essential. It eliminates duplication and outdated information, driving informed decision-making and lower administrative costs. This approach also provides a streamlined audit trail in the event that a regulatory agency raises a product safety issue. For example, if a bad lot of drug compound is released into the market, a pharmaceutical manufacturer can quickly establish where the lot was manufactured, which equipment was used, the source of the ingredients, and the locations to which the compound was distributed. A single version of truth, which is helpful in all industries, is especially critical in regulated industries because it eliminates the need to synchronise multiple sources of redundant data and manage a host of different technologies—which increase risk and complexity.
Process and workflow automation—which allow organisations to build quality into the process—may be IT’s single greatest contribution to enabling a Lean Six Sigma environment. Integrated IT infrastructures allow pharmaceutical manufacturers to rely less on manual checks, which present greater risk and variability, and more on automated checks that are built-in, enforced going forward, and can easily be audited by the FDA and other regulatory agencies. For instance, automation enables manufacturers to enforce electronic signature checkpoints during the processing of a production batch order and automatically notify key personnel of nonconformances, so that reviews and action can be undertaken quickly.
The capture and processing of in-line data is critical to a Lean Six Sigma environment—as well as PAT—because manufacturers must understand all sources of product variability. This understanding cannot be achieved without collecting data from every part of the supply chain and manufacturing process. For example, a manufacturer’s probe sensor might sample the particle size of a batch of product during a granulation process. To make adjustments that would improve product quality or consistency, the manufacturer must determine how the particle size compares to previous batches and standards—a process that depends on the capture and analysis of in-line and benchmarked data—and understand how various possible process adjustments will impact all critical technical attributes of the material. An integrated IT infrastructure is essential to enabling manufacturers to capture the secure, analysable and actionable data needed to transform their operations.
Electronic record keeping plays an important role in helping pharmaceutical manufacturers build quality into the process. Paper records are cumbersome and expensive to circulate for review and approval when there are multiple staff members or departments involved in the process. This challenge is compounded in a global
enterprise. Faster and cheaper product development, manufacturing and quality assurance turnaround is possible with electronic routing of signature requests anywhere, virtually and instantly. Electronic records also improve accuracy. There are limited means to prevent users from entering invalid data on paper forms beyond rigorous and time-consuming manual checking. Sophisticated electronic record systems, however, are adept at reducing data errors by providing users with lists of appropriate values from which to choose, and by validating data formats prior to accepting or saving the data into files or tables.
Pharmaceutical manufacturers possess massive quantities of data on processes as far ranging as purchasing of office supplies and analysis of data from gas hromatographs. Many, however, cannot analyse or interpret paper-based or siloed information to identify important trends and drive improved manufacturing practices.
A single source of truth, coupled with advanced analytics, enables pharmaceutical manufacturers to run real-time analysis that yield the kind of business intelligence that reduces risk, helps to improve operating efficiency and agility, and streamlines compliance. For example, a manufacturer can use advanced analytics to conduct quality analysis, risk assessments, yield analysis, on-time production tracking, scrap reason analysis, cost comparisons by job, and comparisons of manufacturing plans and efficiencies between sites, to name just a few of the endless possibilities.
Removing variability in processes and materials is fundamental to a Lean Six Sigma environment. IT systems provide the information necessary to establish an environment that supports risk-based decisions. IT serves as a lens through which processes can be observed, monitored and measured. Only then, can manufacturers enable greater control over variability.
Process automation further enhances operational efficiencies. On the materials management front, an integrated IT infrastructure drives automation that enables manufacturers to enforce business rules that require materials to go through certain quality tests before they reach a customer, as opposed to relying on a paper document to confirm a test has been completed. Automating controls also reduces ongoing complexity, redundancy, the potential for operator error and, ultimately, waste.
Leading IT vendors, such as Oracle, are removing the complexity from process integration by leveraging Business Process Execution Language (BPEL), which allows manufacturers to build a process once and then apply it throughout the environment. BPEL is emerging as the standard for assembling a set of discrete services into an end-to-end process flow, radically reducing the cost and complexity of process integration initiatives.
A demand-driven model, which supports Lean Six Sigma principles, allows pharma manufacturers to postpone inventory build-up and reduce inventory carrying costs and the risk of a product expiring before sale. The transition from a make-to-stock approach to a demand-driven environment, however, has not been an easy one for many manufacturers.
Most companies today have a fragmented process for Sales and Operations Planning (S&OP). Each department tends to have its own process with critical company data stored on spreadsheets. Departmental plans are not aligned, and there is misalignment between how departments are measured and overall company objectives. For example, sales management is often measured on meeting a sales quota that may be achieved by selling products that the supply chain is unable to produce. This tends to lead to a very time-consuming and manual process of trying to come to agreement on “the forecast.” This painful exercise typically yields an inaccurate forecast. The forecast is then “tossed over the wall” to the supply chain to figure out how to expedite processes to meet the demand with no thought given to the profitability of the decisions. Further complicating the process is the fact that the “approved” plans, which may exist on spreadsheets, are often filed away, and have little relation to the actual plans being executed.
New IT solutions can help manufacturers address their complex S&OP needs by enabling them to bring all business areas together for the purpose of aligning supply with demand and delivering an operational plan designed to achieve a defined corporate business strategy. Some systems, for example, allow a direct linkage between sales orders and production batches, allowing users to create a batch reservation for a sales order. When the batch is completed, the reservation for the order line is converted into an inventory allocation and can then be confirmed and shipped. Alternatively, if there are no existing batches planned or in process for the required product, a user can initiate a request to create a batch specifically for that order. Automated workflow notifications keep the order entry personnel apprised of any changes to the production schedule that may impact their order.
Pharmaceutical manufacturers often have multiple manufacturing plants, warehouses, distribution centres, and transportation lanes and modes. Determining the best manufacturing, distribution and logistics choice becomes an exercise in selecting from among thousands of combinations. Dynamic inputs to the network design process—such as fuel prices, currency exchange rates, real labour rates and seasonal demand—further complicate the process. Advanced Strategic Network Optimisation (SNO) solutions can help manufacturers optimise choices and combinations. SNO solutions perform two distinct functions, simulating and optimising different supply chain configurations and creating dynamic sourcing rules to be used by downstream planning processes. These solutions, which combine a flexible supply chain modelling environment with highly tuned solver algorithms and visualisation capabilities, allow users to define, simulate and evaluate complex manufacturing, distribution and transportation supply chain problems—before making costly mistakes.
It is very difficult to determine corrective and preventative actions without a sound understanding of variability sources and estimates. The information required for a corrective and preventative action includes appropriate details of the event, the time and date of the nonconformance, the phase of the batch in which the nonconformance occurred, details of the incident or observation, level of criticality, and required follow up, as well as the signatures of various operator(s) and / or supervisors. This process, which is essential to both quality improvement and regulatory compliance, is costly and time consuming when completed manually.
It also presents many opportunities for data omission or the recording of incorrect data. Corrective and Preventive Action (CAPA) solutions manage issues to closure through an automated workflow, and provide appropriate documentation required for regulatory compliance.
Pharmaceutical manufacturers are on the cusp of realising the benefits that Lean Six Sigma practices can deliver to their organisations and the industry as a whole. To successfully transform their organisations, however, pharmaceutical manufacturers require greater visibility into their end-to-end operations—an objective that cannot readily be achieved through paper-based processes or disparate IT systems. To this end, pharmaceutical manufacturers increasingly look to integrated IT infrastructures to help them execute Lean Six Sigma paradigms and, ultimately, achieve new levels of operational efficiency, quality and corporate performance.