Understanding the link between maturity and performance provides a competitive advantage for companies on their way to continuous improvement. This article illustrates the St.Gallen operational excellence (OPEX) model’s concept of site performance and maturity, bringing both perspectives together in a holistic framework.
Pharmaceutical plants are increasingly concerned with improving their respective maturity. The numerous initiatives are labelled with terms like ‘lean maturity assessment and improvement’, ‘Quality Management Maturity Improvement’, etc. What is less clear, though, is if this will have a visible impact on the performance. Plant performance has always been and will always be in the centre of management attention. The true performance of a system cannot be measured with isolated metrics, but requires a holistic, balanced approach to be assessed and managed (Friedli & Bellm, 2013). In 2002, the U.S. Food and Drug Administration (FDA) announced the pharmaceutical current good manufacturing practice (cGMP) for the 21st Century initiative. Goal of the initiative was to encourage industry in adopting modern and innovative manufacturing technologies to improve quality, performance, and patient security within the pharmaceutical industry (FDA, 2004). As results, lean thinking and OPEX became not only a suitable instrument to improve operations but also regulatory expectations (Friedli & Bellm, 2013).
FDA’s efforts to shift operations in the pharmaceutical industry from a lagging state of OPEX implementation to a more mature and efficient state have left a mark (Eich et al., 2020). In fact, the industry has invested numerous resources in developing some of the most sophisticated OPEX production systems in the world (Friedli et al., 2013). These investments did not only improve quality but also generated a pay-off in better overall manufacturing performance. This article discusses the relationship between plant maturity and plant performance. Firstly, both concepts of performance and maturity are introduced. Successively, the operationalisation of the two elements is explained based on the St.Gallen OPEX model. Finally, the link between performanceand maturity is analysed and presented.
Since the development of the DuPont system in the 1920s, many different key performance indicator (KPI)-systems have been presented, of which some are also discussed as competing approaches of the same origin. With the criticism of such traditional KPI-systems emerging in the 1970s and 1980s, the performance measurement revolution was initiated (Eccles, 1991). This revolution led to the development of several new approaches which, in contrast to traditional KPI-systems, consider performance from a broader perspective (Bourne et al., 2000). Therefore, performance measurement does not only refer to financial or past-oriented indicators but rather can be understood as the structure and use of several KPIs from different dimensions such as costs, time, quality, innovation capability or customer satisfaction (Kaplan & Norton, 1997). These dimensions are then used to measure and evaluate both the effectiveness and efficiency of a system (Gleich et al., 2011). A major advantage of this holistic view is that dysfunctional behaviour in favour of a short-term or unit-based view is avoided (Bititci, 1994; Neely et al., 1997). This implies that for long-term success, production sites should measure performance on a holistic level and not only on single KPI level (Friedli & Bellm, 2013).
While performance is an outcome measure concerned with effectiveness and efficiency, maturity is focused on capability building and enabling of the organisation. Capabilities are comprised of resources, such as skills and tools, and processes in place that in turn enable and build the basic requirements to achieve effectiveness and efficiency (Wu et al., 2010). From our point of view, maturity reflects the degree to which an organisation has implemented skills, tools, methods and processes and concurrently has a supporting underlying culture. Ferdows and Thurnheer (2011) use an analogy from the sports world to describe this phenomenon: factory fitness. The theory behind factory fitness is that excellence in production is built on a common set of core capabilities. These core capabilities however are easier to be developed starting with one specific type of activity followed by another. Accordingly, each initiative facilitates the implementation of the next, and each subsequent activity extends and enriches the effectiveness of the previous activities (Ferdows & Thurnheer, 2011). Plant maturity in that sense refers to a set of capabilities that need to be developed in a stage-like and sequential structure. For a class of objects, usually consisting of numerous attributes, discrete stages describe an improvement and development path from an initial state to a target state. The lowest level represents an initial state, which may be characterised, for example, by the fact that an organisation has few capabilities in the area under consideration. In contrast, the highestlevel stands for world class maturity. In order to progress on the evolutionary path between the two extremes, a systematic development of capabilities, behaviours and processes is required. (Becker et al., 2009) The own starting point on that path is usually defined with the help of maturity assessments. These assessments typically consider different dimensions and areas in a plant, in which the specific characteristics and data are measured with Likert-Scales or binary queries. Maturity assessments are usually done with internal or external audits including the use of questionnaires or checklists.
Several excellence models, such as the European Foundation for Quality Management (EFQM) one’s, have been developed to describe the way to Excellence. All these models have some commonalities. They heavily focus on leadership for organisational improvements, consider people as a resource, and integrate processes (Friedli & Bellm, 2013). Nevertheless, in the case of the pharmaceutical industry, the integration of subsystems is not straight forward because of a tendency to avoid the quality system when striving for OPEX. Moreover, some OPEX models and programs are mistakenly seen and accordingly managed as costcutting exercises. The St.Gallen OPEX model (see Figure 1), however, follows a holistic OPEX approach aiming for true integration. Its operationalisation is described below.
The model reinforces the importance of equipment and process stability to sustain product quality (Friedli & Bellm, 2013). It can be divided in two sections, on the top the technical subsystem focussing on the practices and techniques necessary to achieve OPEX. Simultaneously, the bottom part reflects the social sub-system which summarises leadership behaviour, employee empowerment, and work organisation. Figure 1
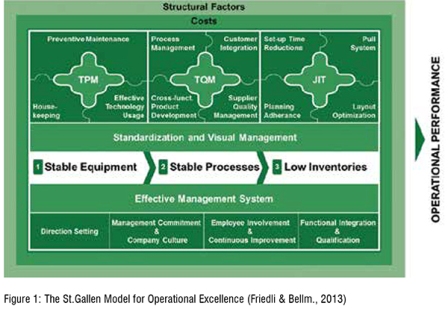
The technical sub-system is further structured in three major widespread operations management principles, total productive maintenance (TPM), total quality management (TQM) and justin- time (JIT). The model depicts the correct order in which principles should be implemented, starting from the left to the right. Starting with ensuring stable equipment, the plant can then aim at stable processes and eventually reduce inventories and streamline operations eliminating waste, all contributing to superior operational performance. The first step is to improve TPM practices, promoting overall effectiveness by creating comprehensive productive maintenance system and maximising equipment reliability. Successively, the TQM philosophy will bring in continuous process improvement by connecting the value chain from supplier quality to customer requirements. Finally, JIT aims to reduce waste in order to establish the ‘zero-waste’ system and reduce all non-required elements. An additional technical sub-system element is provided by basic elements (BEs). BEs summarise all practices that are shared by TPM, TQM and JIT and that are not unique to either of the principles.
The social sub-system is divided in four central principles with the objective of describing how to motivate and align people to reach a common goal. To ensure the alignment, it is fundamental that the leaders formulate a strategy and provide direction setting to the employee. Moreover, to facilitate the change process management commitment is required. Management must promote a company culture which supports people in doing and improving their work. The next step is to guarantee employee involvement and continuous improvement, creating buy-in from all the employees and getting their willingness to improve. The last social principle is functional integration and qualification. Employees need multiple skills and knowledge based on the different technical principles to eventually support and bring continuous improvement to life.
Hayes and Wheelwright (1984) compared the competitiveness of manufacturing companies in the United States, Europe and Japan. The result of the study showed that the superiority of Japanese companies is due to superior production capabilities. Thereafter, the world class manufacturing (WCM) project was launched to identify critical factors of successful manufacturing companies. Based on the WCM project, two crucial dimension structure OPEX. The first one refers to the effectiveness of a production system, the second to the effectiveness of applied approaches and practices (Hayes & Pisano, 1994). Building on the WCM project’s results, the St.Gallen OPEX model measures both performance and maturity of the practices comprised in the technical and the social sub-system. On the one hand, the performance of the technical sub-system is measured in the dimension of TPM, TQM and JIT with the help of numerous KPIs. The performance of the social sub-system is operationalised under the term of effective management system (EMS) and measures the performance of the social practices. On the other hand, the maturity of the practices is measured with the help of enablers questions in all practices again structured along of the model’s categories TPM, TQM, JIT, EMS and BE. The measurement relies on a five-point Likert Scale system, where each stage of the scale is matched with an exact description of the respective maturity degree. This operationalisation of both KPIs and enablers in certain dimensions allows to identify specific performance gaps, respective levers to pull, and to derive the right implications.
The last system in the St.Gallen OPEX model is the structural factors system. Structural factors describe the specifics of a plant, for example product type, size, and complexity. The consideration of the structural factors ensures a holistic picture and the right interpretation of a plant’s current situation.
Performance and maturity should not be analysed in isolation, which is why the St.Gallen OPEX model integrates both dimensions. Literature suggests a direct link between performance and maturity and poses the hypothesis of higher adoption of practices, namely maturity, leads to better operational performance (Voss et al., 1995). In addition, it is suggested that the implementation of practices should not follow a piece-meal investment approach but a rigorous, coordinated and integrated deployment approach (Schoenherr & Narasimhan, 2012).
The link between maturity and performance can be best analysed in a framework that spans two dimensions: x-axis for maturity and y-axis for performance. Voss et al. (1995) conducted a four-country study that investigated the competitiveness of manufacturing sites in the respective countries, using such a framework to cluster the participating sites. Thereby, they came to two conclusions. On the one hand the four countries differed in their competitiveness because their sites had different performance as well as maturity levels. On the other hand, there was a positive correlation between performance and maturity.
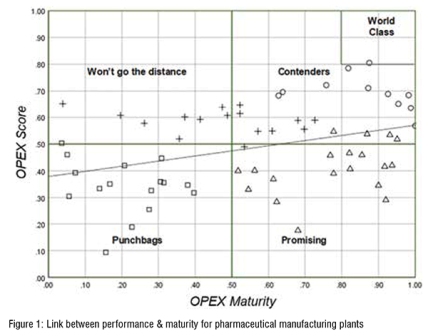
We examined the link between maturity and performance in pharmaceutical manufacturing plants. Our data stems from the continuous St.Gallen OPEX Benchmarking, initiated in 2004, and currently counting data from more than 380 pharmaceutical manufacturing plants. A questionnaire, structured and operationalised with the St.Gallen OPEX model, is used to gather data from the sites. We aggregated an OPEX performance score based on the comprehensive KPI set and an overall OPEX maturity score based on the enabler questions, to map and cluster the sites in the Voss et al. framework1. The results of this analysis are shown in figure 2.
Our analysis reveals a positive correlation between maturity and performance. That is, higher maturity of a plant is associated with higher performance of a plant. Some plants, however, do not fall into this pattern. One cluster shows low performance with high maturity (Voss et al. called this cluster ‘promising’) and another cluster shows high performance with low maturity (named by Voss et al. as “won’t go the distance”). A possible explanation for the former is that these sites have just started their OPEX journey and the implementation will pay off soon or their approach does not follow an integrated approach (cf. Schoenherr & Narasimhan, 2012). Sites in the latter cluster might have gained performance advantages by a short-term optimisation aggressively aiming at cost-reduction. Yet, their performance is not built on a profound and sustainable foundation, which is why they are expected to drop in their performance level eventually.
The integration of various dimensions in performance measurement provides an exhaustive picture of the effectiveness and efficiency of a pharmaceutical plant. Performance is seen as the outcome from a plant’s OPEX maturity. The St.Gallen OPEX model brings both perspectives together and offers the possibility to holistically assess maturity and performance. An external benchmarking provides a profound baseline for continuous improvements. Our forthcoming article will provide more insights into the setup of a meaningful benchmarking and will demonstrate how the pharmaceutical industry evolved in its OPEX approach over the years.
Reference:
1 Our aggregation logic will be further explained in a forthcoming publication in Pharma Focus Asia.