Automation platforms that can be easily expanded to other process automation applications within the manufacturing facility would significantly reduce cost involved in ensuring regulatory compliance.
The global production value of the pharmaceutical industry is expected to grow from US$ 370 billion in 2000 to about US$ 660 billion by 2010. The pharmaceutical industry is in rapid transition from a supply-driven market to a demand and service-driven market where manufacturing efficiency and responsiveness will play a critical role in future success.
Manufacturing is evolving from a high margin, large volume, make-to-stock, supply-driven operation to a price sensitive, small volume, flexible, make-to-order, personalised, value-driven services operation. With limited available capital, equipment, and talented human assets, maximising asset utilisation and return on assets is becoming vital to future success and survival. Companies are building manufacturing cultures that foster innovation and teamwork while utilising common tools, technologies, and standards that deliver added value and financial benefit to their businesses.
Outsourcing driving the need for regulatory compliance
Global pharmaceutical majors are under immense pricing pressure. As a result, they are resorting to outsourcing work to Asia to ensure cost savings. They are trying to establish their presence in countries such as China and India to take advantage of the lower costs. In order to comply with stringent FDA norms and GMP, as well as, achieve global standards, maintain uniform quality, pharmaceutical manufacturers in Asia are resorting to implementing automated solutions.
Manufacturers world over in general, and Asia in particular, suffer from the existence of islands of automation in their manufacturing facility. State-of-the-art automation platforms/systems that can be easily expanded to other process automation applications within the manufacturing facility would significantly reduce cost involved in ensuring regulatory compliance.
Part 11 of the Title 21 of the Code of Federal Regulations (CFR); Electronic Records: Electronic Signatures (21 CFR Part 11) stipulated by the FDA, applies to records in electronic form that are created, modified, maintained, archived, retrieved or transmitted under any records requirements set forth in the agency regulations. 21 CFR Part 11 requires pharmaceutical manufacturers to provide greater production transparency through audit trail and access control functions. It also puts forth the criterion that electronic records and signatures are equivalent to paper records and handwritten documents in the manufacturing processes. Therefore, companies are compelled to develop sophisticated means of electronic validation and batch recording.
Electronic Batch Record Systems (EBRS) are a perfect solution for the pharmaceutical industry. Through a userfriendly Graphic User Interface (GUI), EBRS would provide an efficient way for automatic capturing of data, exchange of batch information, batch production management, and report generation, to increase productivity and accuracy of operators. EBRS would also provide a central storage of data to maintain data security and integrity. By providing functionality for application security, audit log generation, and e-Signature capture, EBRS would ensure that the system becomes completely compliant with the 21 CFR Part 11 regulations.
Process Analytical Technology (PAT): It is a framework for innovative pharmaceutical development, manufacture and quality assurance. Through this framework FDA is encouraging pharmaceutical manufacturers to adopt innovative technologies without fearing validation risks and production delays. This in turn is creating a demand for automation solutions. A desired goal of the PAT framework is to design and develop well-understood processes that will consistently ensure predefined quality at the end of the manufacturing process. Such procedures would be consistent with the basic principle of quality by design and could reduce risks to quality and regulatory concern while improving efficiency. Increasing automation to improve operator safety and reduce human errors will ensure gains in quality and efficiency. Vendors in the plant automation space illustrate how their products can help a manufacturer work towards PAT goals. A PAT environment would comprise of analysis, data mining, quality systems, documentation, implementation, validation and regulatory compliance. Some of the Manufacturing Execution Systems (MES) solutions help in addressing the PAT requirements. A broad range of tools and technologies like infrared spectroscopy systems, a variety of sensors, advance process simulation tools as well as historians can be employed by pharmaceutical manufacturers to make their product features more predictable.
ISA-88 standard for automation structure: Pharma manufacturers are under pressure to innovate. The competition from generics is urging companies to be flexible in manufacturing pharmaceuticals. Hence, companies are forced to manufacture potent drugs in smaller volumes. This necessitates the production of multiple products on the same equipment.
Smart manufacturers leverage the primary standard ISA-88 for achieving improved quality and enhanced operational excellence. Among the 150 standards published by ISA, a non-profit organisation globally involved in writing and developing standards, ISA-88 standard is for flexible manufacturing and batch control. ISA-88 is the accepted standard for batch processes.
It enables flexible automation. ISA-88 standard defines the plant floor automation structure and is an excellent tool for definition of automation requirements. ISA-88 standard defines a common set of models and terminology that can be used to describe and define batch-manufacturing systems in accordance with GMP. Pharmaceutical manufacturers would benefit immensely from incorporation of ISA-88 methodology for its batch control systems.
As per the latest US FDA list, 14 Indian companies have received 77 approvals or tentative approvals for active ingredients during January to November 2006 as compared to 60 approvals by 9 companies in the year 2005. Out of the 77 US FDA approvals for active ingredients, 31 were tentative approvals and remaining 46 were final approvals. The Indian companies are well set to obtain more approvals in the coming years and are investing in expansion of the manufacturing facilities. Similarly pharmaceutical manufacturers in other parts of Asia, China in particular, are investing in expansion and upgradation programmes, which will enrich their product pipeline in the coming years.
This expansion and upgradation activity would necessitate implementation of Process Automation Systems (PASs), whether PASs are part of a new plant construction, an upgrade to an existing facility, or a long-planned plant expansion, deciding how to choose and implement them takes time, money and plenty of control expertise. They can be implemented through a system integrator and / or in conjugation with a PAS supplier. Employing consultancy firms will help to avoid vendor bias and aid in the right choice of the systems.
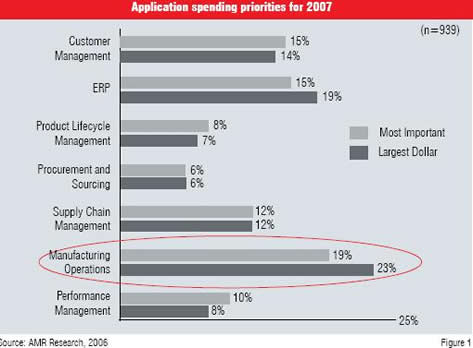
A survey conducted by AMR Research indicated that applications related to manufacturing operations are the top most priority in the IT spend of manufacturers for the year 2007 (refer Figure 1). Pharmaceutical manufacturers in Asia like those in the rest of the world are feeling the economic pressure. Most of the manufacturers are striving for reduction in down time and manufacturing cost, improved time to market and compliance to regulations. The market for automation in the pharmaceutical industry in Asia has grown from US$ 700 million in the year 2000 to US$ 950 million in the year 2005. It is estimated to reach US$ 1450 million in the year 2010. This clearly reflects the strategy of the pharmaceutical manufacturers in Asia to implement plant automation systems that would ensure enhanced operational excellence.
Acknowledgment
The author acknowledges the support extended by Dr. Norbert Schroeder of INTECHNO CONSULTING, Switzerland in providing facts and figures for the above article.
1. Pharmaceutical plant markets 2010, Global market analysis and forecasts until 2010, Intechno Consulting, Basel, Switzerland.
2. United States Census Bureau
3. ARC Advisory group reports
4. Pharmabiz.com reports
5. Control Engineering Asia reports
6. AMR’s research articles