Quality risk management plays a key role within pharmaceutical production. Being able to predict quality issues and proactively maintain regulatory compliance prevents production downtimes and disruptions of medicine supply. This article outlines a comprehensive approach how to address and predict quality risks by utilising internal and external metrics.
Proactive quality risk management provides numerous advantages to pharmaceutical companies, such as less rejected batches, and less drug shortages (FDA, 2021a). It helps to minimise and predict potential negative impact on patients caused by quality issues, and it encourages manufacturers to adopt suitable continuous improvement programs. The combination of quality standards and good manufacturing practices constitutes the guidance for industry ICH Q10 Pharmaceutical Quality System (PQS), which sets the basis to establish a holistic quality system and a continual improvement of product quality (ICH, 2008). Academics addressed quality risks in pharmaceutical production with a special focus on two subject areas: analysing the methods and tools for managing and reducing quality risks in the processes (Altamuro et al., 2017; Ball et al., 2017; Seiss, 2018) and identifying context factors influencing quality risk at the plant level (Gray et al., 2015a; Gray et al., 2015b; Gray et al. 2016).
However, both industry and academia are lacking a comprehensive approach to measure, operationalise or quantify quality risk. Regulatory programs, such as the quality metrics initiative showed that the effectiveness of a PQS cannot be assessed based on a few single key performance indicators (KPI) (Friedli et al., 2019). Similarly, a quality risk assessment should consider the site's characteristics, operational data sources, and external data sources (ICH, 2019). Moreover, the Covid-19 pandemic has shown that also regulatory authorities might be forced to perform remote inspections and prioritise oversight activities (FDA, 2021b). These newly raised regulatory efforts to better evaluate quality metrics and risk underline the importance of predicting instead of reacting to potential menaces to quality.
Predictive quality risk assessments can address the needs of different parties: pharmaceutical companies benefit from improved quality systems, regulators can ensure oversight in a more efficient way and academics apply new methods to an established field of research. Discussing predictive quality risk assessments in the following, we will differentiate between internal and external quality risk perspectives. Internal perspective describes factors related to the manufacturing site while the external perspective is connected to the environment, which is surrounding the establishment. We will present early findings of researching the internal perspective on predictive quality risk assessments, outline approaches for addressing the external perspective afterwards, and underline the importance of integrating both subsequently. Lastly, the article will provide an outlook to future research activities in this area.
Manufacturing facilities are routinely inspected by authorities to minimise patients' exposure to unsafe drugs. The U.S. Food and Drug Administration (FDA) already assesses risk to schedule inspections and identify firms that may incur quality violations and prioritise these for inspections. FDA's risk assessment is based on the site type, time since last surveillance inspection, FDA compliance history, foreign regulatory authority inspectional history, patient exposure, hazard signal, and inherent product risk (FDA, 2018). Combining the compliance history with broader risk factors is expected to be a key lever to improve risk forecasting accuracy. Seiss (2018) showed how compliance history data does help to predict future inspection outcomes, especially if combined with additional parameters. For this reason, the FDA aims to leverage additional data in combination with compliance history to optimise the inspection schedule (FDA, 2021b).
The selection of appropriate data to combine with compliance history is a crucial element for better quality risk prediction. Thus, different parameters and signals must be analysed to reveal telling risk predictors. According to the ICH (2005), contributions to quality risk management at the plant level should cover all steps of the value chain and all stages of a drug's lifecycle. A simple consideration of a single production process or department is, therefore, not sufficient to determine the quality risk entirely. A comprehensive plant quality risk assessment shall consider both manufacturing and quality control (QC) lab testing processes. In addition to fulfilling its role as a safeguard for product quality and adherence to specifications, robust quality control is crucial to ensure reliable and timely release of medicines to supply the market (Ritz, 2021).
Site’s quality risk assessment can be operationalised by detailing two major dimensions: Quality maturity and performance. At the same time, these two dimensions provide a categorisation of different sources of risks. Moreover, they provide a structure for the different data sources to subsequently characterise a predictive model.
A site’s Operational excellence (OPEX) maturity is an umbrella term that summarises all initiatives driving continuous improvement along the manufacturing chain (Friedli & Bellm, 2013). OPEX initiatives have been started in the pharma industry for several reasons, one of them is to directly improve process and product quality. The implementation of lean concepts provides advantages in terms of operational performance and enables plants to achieve better results in quality if implemented correctly. In the final report after supporting the Quality Metrics Initiative for three years, Friedli et al., (2019) analysed the relationship between OPEX practices (defined as Overall Enabler, Total Quality Management Enabler, Just-In-Time Enabler, and Quality Maturity) and compliance status of pharmaceutical production facilities. The results are displayed in Figure 1, inspection outcomes are clustered in two categories: No Actions Indicated (NAI) and Actions Indicated (AI) comprising of voluntary and official actions indicated. Within all four diagrams, sites showing a maturity level below the median in the respective category reveal a higher amount of AI results compared to the sites above the median. The implementation of higher maturity in all categories provides confidence for a statistically significant relation with NAI inspection outcomes. (Figure 1)
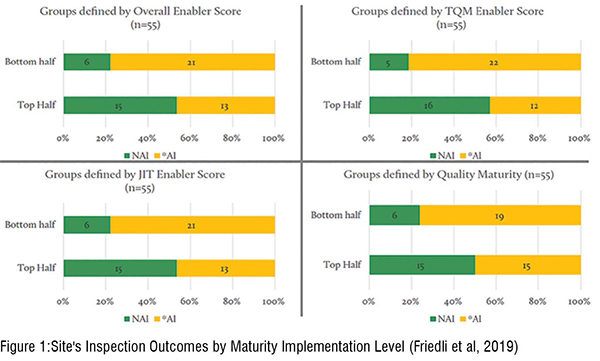
Subsequent research confirmed the preliminary findings of Friedli et al. (2019). Eich & Friedli (2021) disclosed that sites with a higher implementation of OPEX practices, especially in total quality management, show better inspection outcomes. Another important internal signal that is leveraged by FDA (2018) and mentioned by ICH (2005) is the inherent product risk. Drug products carry a different level of inherent risk depending on their characteristics. For example, large molecules products are more complex and carry higher risks compared to small molecule products (Basu et al., 2013). Moreover, a product's risk is influenced by its lifecycle stage. A product in the initial phase does carry a higher inherent risk per definition compared to a very established medicine.
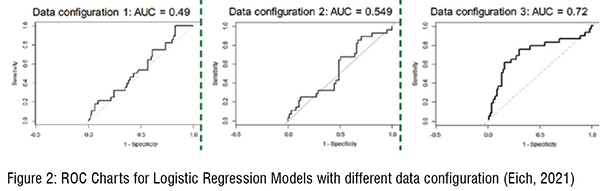
An additional font of information about current quality status at the site level can be drawn from operational performance metrics. Eich (2021) demonstrated that the integration of operational KPIs improves the predictability of the risk assessment providing a better capability of forecasting the outcomes of compliance inspections. Figure 2 shows how the reliability of the logistic regressions improves by adding new parameters to the model. Starting only from the compliance history (left) the model is not too reliable, by adding the product type (middle) the model is improved. Finally, by combining compliance history, product type and operational KPIs (right) the model soundly predicts inspection results. The analyses support the overarching idea of integrating manifold perspectives when designing sufficient predictive quality risk models.(Figure 2)
As previously discussed, early attempts to assess quality risk and predict future inspection outcomes have mainly focused on the manufacturing site level. The state of the art on data-based quality risk evaluation at the site provides some insights on how different parameters are related to inspection outcomes. The sole quantification of internal related risk would not be sufficient to provide a comprehensive plant risk assessment because of the numerous external risk factors. External drivers for quality risk have been analysed less, if not almost ignored regarding the pharmaceutical industry. Therefore, considerations on external signals can only be based on initial reflections. Research in this particular field was recently started and is among the priorities of the OPEX team at the university of St.Gallen.
Since every manufacturing facility is connected with the external environment, the external originated risk might impact the internal production processes. Supply chain issues are one major cause of drug shortages and raise the risk for patients (FDA, 2019). Further external factors might have a critical impact on a plant's risk level but are hardly manageable from a plant perspective: A manufacturing site has only a limited capability of influencing external factors. Nevertheless, the ability to screen external signals in search of possible quality risks for the plant is the key to prepare the site to face and reduce the impact of these events. External signals can be leveraged to be matched with site internal parameters, such as performance or maturity, to support their validity and improve the predictability of the assessment. Additionally, external data can be used to surrogate the consequences of internal process issues which are difficult to measure, such as using data from online employee satisfaction portals to assess the company culture.
External factors can be crawled by analysing publicly available data from the web and searching for specific signals related to the site or products. The research approach is structured as follows: At first, select clusters of relevant information to provide the structure for the data gathering process to follow. Secondly, define an ontology approach, such the Columbia Ontology for Pharmaceutical Engineering (COPE) developed by Remolona et al. (2017). The ontology organises the different information sources and associations to generalise the multiple data sources relevant to the risk factors. Finally, raw data gathered is processed aiming to extract semantically rich information from the multiple data sources and make them appropriate for the particular risk prediction.
The major challenge in managing such an amount of unstructured data is to provide a clear structure that can both reduce complexity but is still flexible to develop numerous scenarios. For this reason, a data lake provides broader opportunities compared to old-fashioned databases (Khine et al., 2019). Using data lake, the researchers can store different types of data in a centralised way, and not just a fraction of the gathered data stored in a structured format in relational databases. Additionally, the data lake offers great flexibility facilitating and supporting the development of statistical models analysing the lake’s content in detail. Nevertheless, it is important to carefully tailor the data lake to its purpose and avoid turning into data swamps due to redundant, incorrect, and wrong classified data.
Once concepts are in place for both measuring and analysing risk from an internal and an external perspective, this information will be integrated into an aggregated score. By calculating an overarching risk score, it is possible to reduce the complexity and provide a clear and user-friendly risk measurement system. The prediction model will rely on a central data lake, where all information is stored and then aggregated to build the final risk score based on context factors. Context factors describe the environment and the specifics of an establishment. They provide the conditions that must be taken into consideration when assessing the risk and therefore are used as a weighting system. Context factors are a fundamental part of the risk model since the consideration of global phenomena level does not provide a sufficient level of granularity to industry socially related phenomena. The specific context of a particular manufacturing site needs to be adequately represented in the model.
Leveraging the contextual factors, the most suitable internal and external data are selected and extracted from the data lake. Successively, machine learning algorithms will combine historical compliance data with the information extracted from the data lake to generate predictive models. Resulting risk models aim to generate insights to understand the contributions of various factors to the final risk score without compromising the accuracy of the predictive model. The goal is to define the minimum set of internal and external signals that must be incorporated to obtain a reliable and suitable quality risk prediction depending on the context factors that have been set.
The St.Gallen understanding of internal and external risk signals while considering context factors supports the development of predictive quality risk models able to calculate different scenarios and adapt in real-time. Following such a system-thinking based approach provides numerous benefits to the different stakeholders. The model will improve companies’ ability to assess current risk levels, better fulfil regulatory requirements and inform management decisions to assign resources to the most urgent initiatives at the right point of time. Regulators will benefit from better inspection scheduling system, based on the current risk and its possible development. Moreover, the transparency within the industry will improve. Finally, research can truly profit from a sharpened understanding of the roles of internal and external signals in influencing and predicting different risk levels in pharmaceutical production.
Building on the experience gained in projects for risk prediction at the site level, the research team currently focuses the efforts on three fields of priority. Firstly, expanding the understanding of inherent product risk parameters and the creation of product health metrics. to identify the right parameters able to depict the current status of different product and therefore its risk for the production site. Secondly, screening and clustering publicly available data to classify sources for external signals. Finally, integrating the signals in a comprehensive predictive model that considers both internal and external perspectives to provide a readyto-use tool industry’s quality system and management professionals.
Literature
Altamuro, J.L.M., Gray, J.V. and Zhang, H. (2017) “Organizational non-compliance: A study of FDA Regulated Industries”, SSRN Electronic Journal, pp. 1–64. http://dx.doi.org/10.2139/ssrn.2658225.
Ball, G., Siemsen, E. and Shah, R. (2017), "Do plant inspections predict future quality? The role of investigator experience", Manufacturing & Service Operations Management: M & SOM, Vol. 19, No. 4, pp. 534-50. http://dx.doi.org/10.1287/msom.2017.0661.
Basu, P., Friedli, T., & Bellm, D. (2013). "The Future of Pharmaceutical Manufacturing". In: Friedli, T., Basu, P., Bellm, D., & Werani, J. Leading Pharmaceutical Operational Excellence: Outstanding Practices and Cases. Berlin: Springer; 2013. pp. 445-464. Eich, S. (2021), “Using Data Science to Find Predictors of Adverse Regulatory Inspections – Enhancing Quality Risk management in the Pharmaceutical Industry”. Dissertation, in printing, University of St.Gallen, Eich, S, & Friedli, T. (2021), "Analysis of the Effects of Operational Excellence Implementation on Inspection Outcomes in the Pharmaceutical Industry: An Empirical Study". Brazilian Journal of Operation & Production Management, Vol. 18, No. 3. Htpps://doi.org/10.14488/BJOPM.2021.021
FDA (2018), "Understanding CDER’s Risk-Based Site Selection Model". U.S. Food & Drug Administration
FDA (2019), "Drug Shortages: Root Causes and Potential Solutions". U.S. Food & Drug Administration
FDA (2021a), "Drug Shortages for Calendar Year 2020 – Report to Congress". U.S. Food & Drug Administration
FDA (2021b), "Resiliency Roadmap for FDA Inspectional Oversight". U.S. Food & Drug Administration
Friedli, T., & Bellm, D. (2013), "OPEX: A Definition”. In: Friedli, T., Basu, P., Bellm, D., & Werani, J. Leading Pharmaceutical Operational Excellence: Outstanding Practices and Cases. Berlin: Springer; 2013. pp. 7-26.
Friedli, T., Köhler, S., Macuvele, J., Eich, S., Ritz, M., Basu, P., & Calanan, N. (2019), "FDA Quality Metrics Research 3 rd Year Report"
Gray, J.V., Anand, G. and Roth, A.V. (2015a), "The influence of ISO 9000 certification on process compliance", Production and Operations Management, Vol. 24, No. 3, pp. 369-82. http://dx.doi.org/10.1111/poms.12252.
Gray, J.V., Siemsen, E. and Vasudeva, G. (2015b), "Colocation still matters: conformance quality and the interdependence of R&D and manufacturing in the pharmaceutical industry", Management Science, Vol. 61, No. 11, pp. 2760-81. http://dx.doi.org/10.1287/mnsc.2014.2104.
Gray, J. V., Roth, A. V. and Tomlin, B. (2016), Contract manufacturing and quality risk: theory and empirical evidence. Columbus, OH. http://dx.doi.org/10.2139/ssrn.2815520.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use – ICH (2005), "Quality Risk Management Q9". available at: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Gui deline.pdf (accessed: 26 July 2021).
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use – ICH (2008), "Pharmaceutical Quality System Q10". available at: https://database.ich.org/sites/default/files/Q10%20Guideline.pdf (accessed: 268July 2021).
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use – ICH (2019), "Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management Q12". available at: https://database.ich.org/sites/default/files/Q12_Guideline_Step4_2019_1119.pdf (accessed: 268July 2021).
Khine, P. P., & Zhao, S. W. (2018), " Data Lake: A New Ideology in Big Data Era”. ITM Web of Conferences, 17 EDP Sciences. https//doi.org/10.1051/itmconf/20181703025
Remolona, M. M., Conway, M. F., Balasubramanian, S., Fan, L., Feng, Z., Gu, T., Kim, H., Nirantar, P. M., Panda, S., Ranabathu, N. R., Rastogi, N., & Venkatsubramanian, V. (2017), " Hybrid Ontology-learning Materials Engineering System for Pharmaceutical Products: Multi-Label Entity Recognition and Concpet Detection. Comp. and Chem Eng., 107 (5), pp. 49-60. https//.doi.org/10.1016/j.compchemeng.2017.03.012
Ritz, M. (2021), "Operational Excellence Across Manufacturing and Quality Control – A Guideline to Avoid Optimizing Silos". Dissertation, in printing, University of St.Gallen.
Seiss, M. (2018), "FDA CDER ORA Site Selection Model Improvement" Pilot Study