The study developed a nanosized vehicle named'proposome' to enhance the skin absorption of drugs. Proposomes are propylene glycol-based liposomes. The study investigated the skin permeation of proposomes loaded with ibuprofen, tofacitinib citrate, rhodamine B, and lidocaine. These drugs have different physicochemical properties, which was needed to observe the differences in skin absorption of drugs loaded in proposomes. The proposomes had an average diameter of 128 to 148 nm, measured using dynamic light scattering. The drug entrapment in proposomes varied between 42.9 and 52.7 per cent at the same total concentration, enhancing drug delivery by 1.4 to 4.0 times than free drugs. The skin absorption enhancement through proposomes was linearly correlated with the physicochemical properties of the loadeddrugs. Confocal imaging also confirmed higher skin permeation using proposome than free molecules. The safety of the proposomes was assessed using bioengineered reconstructed human skin tissue equivalents thatshowed proposome was safe for skin application. The enhancement of skin delivery of loaded drugs depended on the drug's lipophilicity, which affected drug entrapment efficiency into proposomes, and in turn drug absorption through skin.
Topical delivery is the most acceptable route for treating skin conditions as it provides localized and fast effects. However, the drug delivery through the skin is challenged by the skin outermost layer, which is made up of dead cellsand the lipid matrix. This outermost layer is a primary barrier for therapeutics absorption into the skin, allowingonly small and lipophilic molecules to be absorbed. Various approaches have been developed to overcome this barrier to enhance the skin absorption of therapeutics. Enhanced absorption translates into desired therapeutic effect or fast effect.
Lipid-based vehicles have been extensively explored to enhance the skin absorption of therapeutics, where especially nanosized lipid vehicles have shown great promise. The lipid vehicles can also be used with other safe topical vehicles such as glycerol, and propylene glycol (PG), to further improve skin absorption of therapeutics while also improving drugand formulation stability. Recently, a PG-based liposome, namely, proposome, showed that it can enhance the skin absorption of to facitinib citrate, used for arthritis and psoriasis. Tofacitinib citrate has low skin absorption, which delays the onset of therapeutic effect when given topically. However, proposomes improved the skin absorption of tofacitinib citrate by many folds. This finding became the base for the current study to explore proposomes as topical vehicles for other therapeutics and study skin enhancement. The study hypothesized that proposomes could improve the skin absorption of molecules with different physicochemical properties. Moreover, the study established the correlations between skin absorption enhancement and physicochemical properties of molecules loaded in proposomes. The study also assessed the safety of proposomes which is critical for developing new formulations. The safety was assessed using in-house produced bioengineered reconstructed human skin (RHS) tissue equivalents that mimic the native skin tissue.
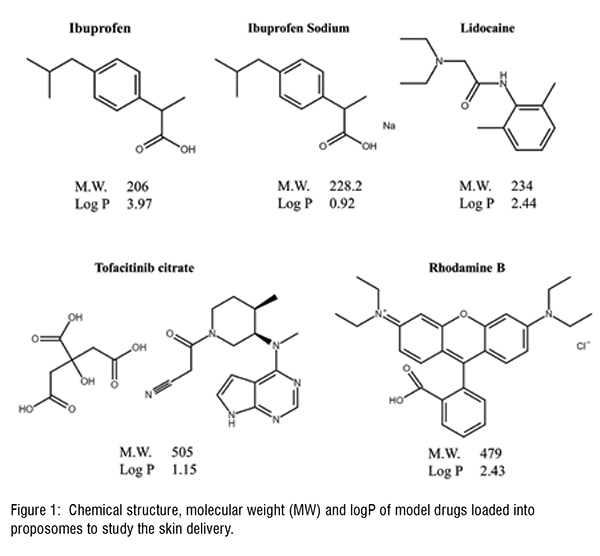
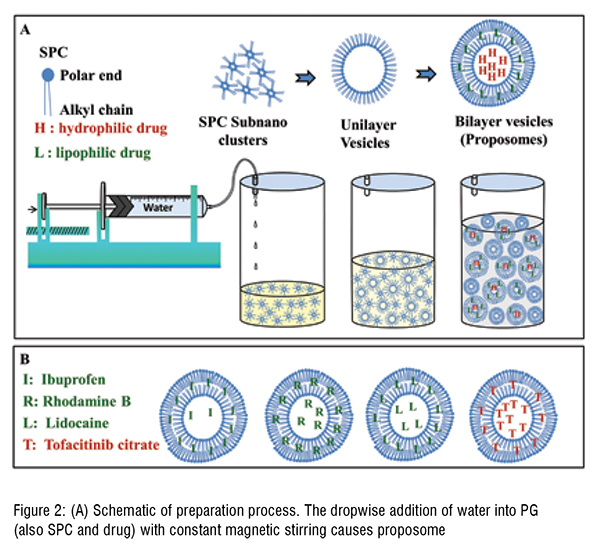
Proposomes were prepared by hydration of lipids dissolved in PG. The hydration was doneunder constant magnetic stirring(~ 1500 rpm) at room temperature and Milli-Q water addition(1 mL/min) using a syringe pump. Proposomes were prepared with 1 per cent soya phosphatidylcholine (SPC), 30 per cent PG, and the following drug molecules were incorporated: Ibuprofen, Tofacitinib Citrate, Rhodamine B, Calcein, and Lidocaine at fixed 5.64 mM concentration. Rhodamine B and Calcein were used as fluorescent probes to observe the skin absorption using confocal laser scanning microscopy (CLSM). The skin absorption was assessed using Franz diffusion apparatus and quantified using a validated analytical method using high-performance liquid chromatography.
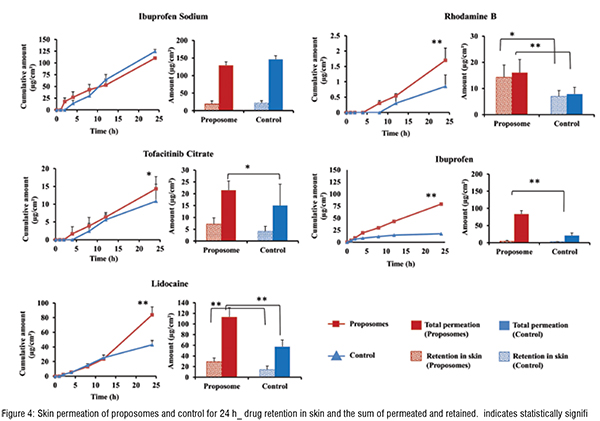
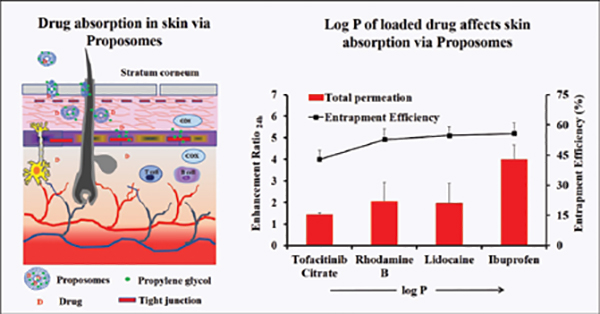
Proposomes sizes were measured using dynamic light scattering. The average size (diameter) of formulations varied between 128 nm to 150 nm, with the narrow distribution shown by a low polydispersity index (less than 0.2). This size is small and can increase skin absorption of molecules. The encapsulation efficiency of molecules varied from 44 per cent to 55 per cent at fixed total concentration. The encapsulation efficiencycan be optimised to increase drug loading by varying the composition of the proposomes and loading techniques. The studies showed that proposomes could enhance the skin absorption of various molecules. The proposome increased both drug permeation through the skin and drug retention in the skin. However, the extent of skin absorption varies depending on drug physicochemical properties. Ibuprofen loadedinto proposome showed at least four times higher skin absorption thanunloaded ibuprofen. While other drugs loaded in proposomes had lower skin absorption than ibuprofen, which depends on their physicochemical properties. Rhodamine B (log P = 2.43, MW = 479.0) and lidocaine (log P = 2.44, MW =234.3) have similar log P while the significant difference in molecular weight; however, both drugs, when delivered with proposomes, showed skin permeation enhancement (two times) close to each other. This could be attributed to the fact that drugs were loaded into the proposomes, changing their skin absorption nature. It showed that drugs with high molecular weight but good lipophilicity could have good skin absorption using proposomes.
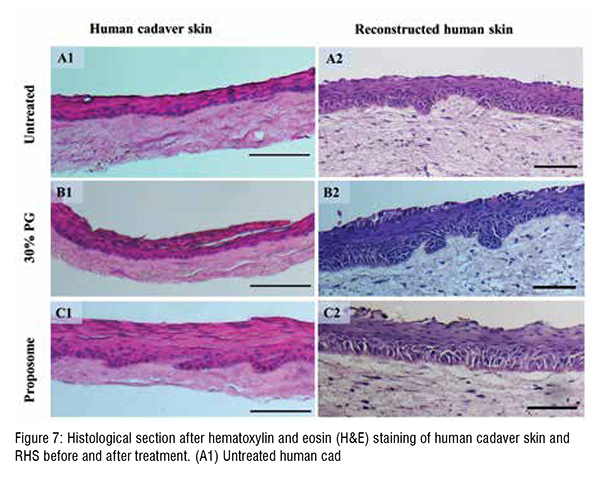
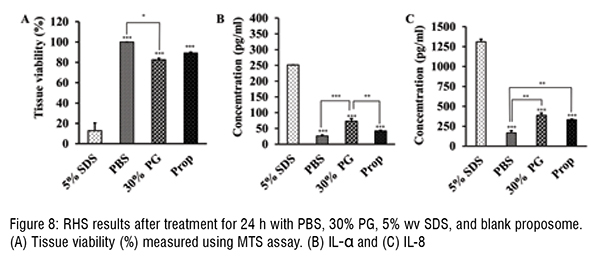
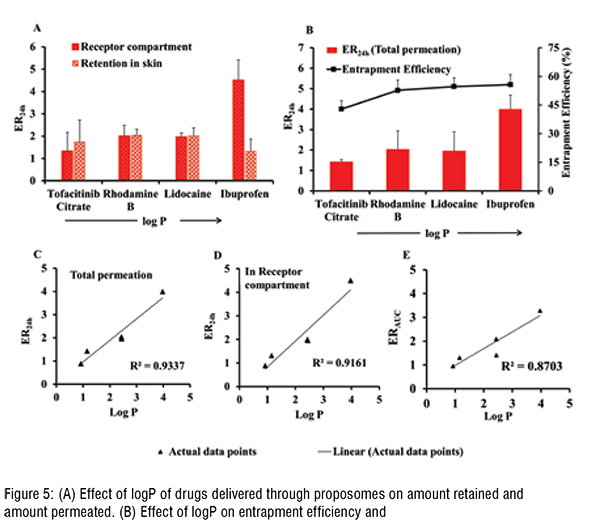
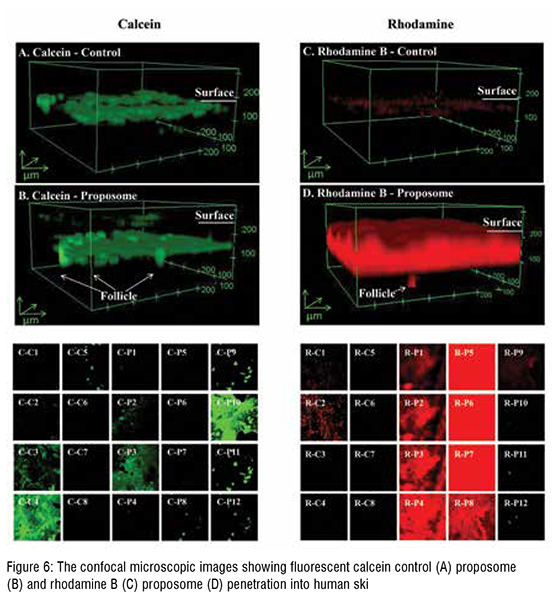
The enhancement and log P showed a positive linear correlation, where this association between log P and enhancement can be explained by entrapment efficiency. The log P of drugs and their entrapment efficiency in proposomes was interrelated, resulting in higher skin absorption.The study shows that even a small increase in entrapment efficiency into proposomes significantly increased skin absorption, which shows the potential of proposomes in enhancing skin delivery. The relationship between drug's physicochemical properties and their skin absorption has been established earlier in literature. However, those relationships are applied to free drugs or drugs delivered without delivery vehicle. In contrast, this study has emphasised the correlation between drug's skin absorption enhancement and drugs loaded in proposomes. Further, skin treated with proposomes oaded with fluorescent molecules was analysed using fluorescent microscopy, which confirmed that proposomes helped in better absorption of both hydrophilic and hydrophobic molecules. Moreover, proposomes helped in deeper absorption of molecules into the skin, which was more than 100 µm deep from the skin surface. Lastly, the safety study shows that the proposomes did not affect the cell viability and did not trigger inflammatory markers in the re-consituted human skin model. These findings that proposomes were non-toxic and safe to human skin.
Conclusion
Proposome is an efficient vehicle for skin delivery of therapeutics, and safe for skin applications.The nanocarriers can increase drug permeation through skin by X folds. X is a number close to the LogP of the drug molecule.
References
Kathuria H, Handral HK, Cha S, Nguyen DTP, Cai J, Cao T, Wu C, Kang L. Aug 2021. Enhancement of skin delivery of drugs through proposome depends on drug lipophilicity.
Pharmaceutics. 13, 1457. DOI:10.3390/pharmaceutics13091457.
Kathuria H, Nguyen DTP, Handral HK, Cai J, Cao T, Kang L. Jul 2020. Proposome for transdermal delivery of tofacitinib. International Journal of Pharmaceutics. 585:119558. DOI:10.1016/j.ijpharm.2020.119558