Pulmonary hypertension is defined as increase in pressure of the blood vessels of the lungs. The mechanism of developing pulmonary hypertension is complex. Recently it has been noted that COVID-19-affected patients are at risk of having pulmonary hypertension. There are already multiple available treatments for this condition. In addition, recent advancements are going on in the treatment of pulmonary hypertension.
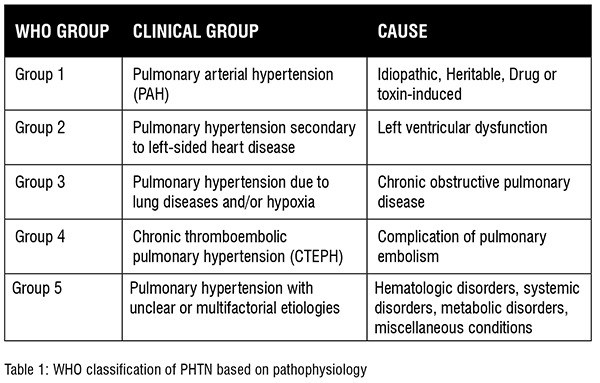
Pulmonary hypertension (PHTN) is defined by mean pulmonary arterial pressure >20mmHg at rest (or > 30mmHg on exertion). The World Health Organization (WHO) has classified PHTN into five groups.
Patients with PHTN may present with multiple systemic involvement and symptoms masking the underlying problem. A high index of clinical suspicion is needed as it is a frequently missed diagnosis due to the non-specific early signs and symptoms. It is necessary to review the management of PHTN as there are still some areas regarding the management of PHTN that need further research and clinical evidence to show positive outcomes.
Currently, there are several therapeutic options available. But there are drugs under trial, and they are presently being evaluated for PHTN treatment. In addition, the recent coronavirus infection 2019 (COVID-19) pandemic has initiated a new interest in PHTN management among health care providers. COVID-19infection has been shown to worsen pre-existing PHTN. COVID-19 is also anticipated to be a significant etiology of PHTN in the coming years due to the long-term sequala of lung fibrosis associated with the infection.
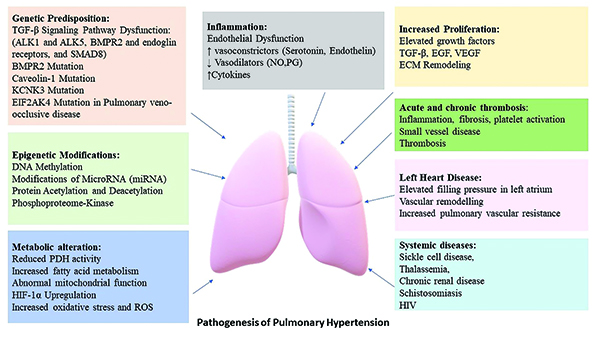
In general, the treatments for PHTN are directed toward the underlying etiology. To fully understand the rationale behind the pharmacotherapeutic options and their benefits, it is essential to discuss the pathophysiology of PHTN. Persistent vasoconstriction, endothelial dysfunction and hyperplasia, remodeling and fibrosis of the pulmonary vasculature, and in-situ thrombosis of the blood vessels contribute to the development of the PHTN. In addition, overproduction of endothelin, reduced nitric oxide production and reduced prostacyclin levels change the delicate balance between the vasoconstrictor and vasodilator mechanisms in the pulmonary vasculature. In PHTN, however, the balance is tilted in favor of the vasoconstrictor mechanism at the expense of the vasodilators. This imbalance perpetuates the underlying disease process, and they form the basis of pharmacotherapeutic intervention in PHTN. Undoubtedly, the development of PHTN is multifactorial. Therefore, it isn't easy to pinpoint a single factor that causes the PHTN as there are multiple factors contributing to the pathogenesis of PHTN that make the treatment challenging.
Recently the COVID-19 pandemic has raised concern for PHTN as a future complication for COVIDaffected patients. Increased production of cytokines such as interleukin (IL)-1, 2, 6, 10, tumor necrosis factor – (TNF- ), and monocyte chemoattractant protein-1 (MCP-1) in the setting of COVID-19pneumonia leads to oxidative stress and subsequent damage to the pulmonary vessels. In addition, the residual effects of the above mechanism can lead to vascular remodeling resulting in PHTN.
The therapeutic target of PHTN has evolved through various animal studies. The result of the studies has guided further clinical trials to find out the efficacy of different treatment options. Mutations of transforming growth factor B (TGF B), bone morphogenic protein receptor 2 (BMPR-2), activinlike kinase type 1 receptor (ALK1), and mothers against decapentaplegic homolog 9 (SMAD 9), endoglin gene, caveolin 1, serotonin transporter gene have shown a role in the pathogenesis of PHTN. Targeting those genes to treat PHTN is an emerging field of development for future treatments for PHTN. Animal studies have shown that 14 days of sacubitril/valsartan treatment has improved the hemodynamic and histological status of PHTN by inhibiting the renin-angiotensin-aldosterone system, increasing natriuretic peptides, and by the anti-inflammatory effect of sacubitril/valsartan. The positive results from the animal studies are the forerunners of future therapeutic options for PHTN patients.
Several medications are already available for the treatment of the PHTN. Voltagegated calcium channel blockers (CCBs) are indicated for patients with PHTN who show a positive response to vasoreactivity testing. A positive response to vasoreactivity testing is defined as a decrease in the mPAP by 10 mm of Hg to an absolute value of less than 40 mm of Hg after administering a short-acting vasodilator agent such as inhaled O2.
The endothelial receptor antagonists Bosentan, Ambrisentan, and Macitentan, are noted to be beneficial in PHTN. Intravenous prostacyclin is another treatment option. Triggering the prostacyclin receptor stimulates pulmonary vasodilation resulting in a decrease in the pressure in the pulmonary vessels. Phosphodiesterase type 5 (PDE5) inhibitors have been shown in clinical trials to improve six-minute walk distance and hemodynamics in PHTN patients. In October 2013, the Food and Drug Administration approved Riociguat to treat adults with PHTN. It is also approved for treating adults with chronic thromboembolic pulmonary hypertension (CTEPH), which is persistent or recurring following pulmonary endarterectomy. Another medication for the treatment of PHTN is Selexipag, an oral, selective, and long-acting non-prostanoid agonist of the prostacyclin receptor. Waxman et al. showed that Treprostinil, initially approved for group 1 PHTN, can be used to treat PHTN in patients with ILD. Inhaled Treprostinil improved exercise capacity from baseline. Thrombogenesis has been proposed to play a role in developing PHTN use of anticoagulation may play a role in PHTN patients. However, clinicians should be careful about possible interactions of anticoagulation medication with other anti-PHTN drugs. The drug interaction can increase the bleeding risk for patients with PHTN. Sotatercept is a fusion protein that binds activins and growth differentiation factors to balance growth-promoting and growth-inhibiting signalling pathways. Humbert et al. reported that treatment with sotatercept reduced pulmonary vascular resistance among patients with PHTN.
There are treatment options available for PHTN, which include nonpharmacologic management. Treatment of sleep-related disorders using continuous positive airway pressure and non-invasive ventilation helps patients with PHTN. The prevention of nocturnal hypoxemia helps alleviate the development of precapillary PHTN. Advances in percutaneous technology opened new possibilities for the management of PHTN. Interventional procedures including balloon atrial septostomy, transcatheter Potts shunt, balloon pulmonary angioplasty, and pulmonary artery denervation have shown promising results in haemodynamic and functional improvements in PHTN patients.
As mentioned before, special attention is required for people with COVID-19 infection as they are at high risk of developing PHTN. For the COVID-19 patients with pulmonary hypertension, inhaled nitric oxide improved blood oxygenation and decreased pulmonary vascular pressure. Puk et al. have shown that endothelin receptor antagonists, phosphodiesterase 5 inhibitors, riociguat, and prostacyclin could help treat PHTN in COVID-19. Clinicians must be cautious while following up on patients with COVID-19 as a low threshold to diagnose PHTN in these patients is important not to miss the complication. Studies are going on to show the effects of sodium-glucose transporter inhibitors to improve pulmonary vascular resistance for patients with COVID-19 infection. Hopefully, the gliflozins will be a future possible treatment option for PHTN, particularly for COVID-19 patients.
In rodent models, mineralocorticoid receptor antagonists have a promising role in preventing or reversing PHTN and right ventricular failure. Decreasing circulating estrogen by inhibiting the conversion of androgens to estrogen using the aromatase inhibitor anastrozole and inhibition of the estrogen receptor with tamoxifen inhibited PHTN in animal models. Tyrosine kinase inhibitors can attenuate platelet-derived growth factor signaling and reduce calcium-sensing receptor expression. This makes the kinase inhibitors to be an effective treatment for PHTN. Inhibition of histone deacetylase and Keratin-1 have the potential to reverse the changes in pulmonary vasculature and PHTN. The role of hypoxia-inducible factor (HIF)2 in the initiation and development of PHTN has been an area of future research. 5-hydroxytryptamine (5-HT) pathway inhibition, activation of vasoactive intestinal peptide (VIP) pathway, pyruvate dehydrogenase kinase (PDK) inhibitors, and targeting the mechanistic target of rapamycin (mTOR) are some areas of limited knowledge that can prove their benefit in the treatment of PHTN in the near future. Results from meta-analysis have shown that stem/progenitor cell therapy is associated with significantly improved pulmonary haemodynamics in animal models. Future studies have scope for exploration of this field.
We cannot ignore the fact that there are still multiple challenges associated with PHTN management. Patients can have multiple types of PHTN together. For example, patients may have heart disease, chronic obstructive pulmonary disease, and connective tissue disorders. The presence of multiple co-morbidities makes the pathogenesis of the PHTN complex and the treatment more difficult. Finding a guideline for treating these patients is often not easy. Most of the clinical trials have excluded patients with a combination of etiologies for PHTN, and thus treatment of these patients is not evidencebased. Dealing with PHTN in patients with idiopathic pulmonary fibrosis (IPF) is also not straightforward. Recently, a multi-centre, international, double-blind, randomised, placebo-controlled, phase 2b study did not show any treatment benefit after adding sildenafil to pirfenidone versus pirfenidone plus placebo up to 52 weeks in patients with advanced IPF and risk of PHTN. More studies are required to find an optimum solution for these complex patients.
Managing patients with PHTN should always be a multidisciplinary decision. There are already different options available for the treatment of PHTN. In addition, newer studies are coming up to show the potential therapeutic benefit of the new drugs/molecules in the management of the PHTN.
References:
1. Hajra A, Safiriyu I, Balasubramanian P, Gupta R, Chowdhury S, Prasad AJ, Kumar A, Kumar D, Khan B, Bilberry RSF, Sarkar A, Malik P, Aronow WS. Recent Advances and Future Prospects of Treatment of Pulmonary Hypertension. Curr Probl Cardiol. 2022 Apr 29:101236. doi: 10.1016/j.cpcardiol.2022.101236. Epub ahead of print. PMID: 35500734.
2. Guazzi M, Naeije R. Pulmonary hypertension in heart failure: pathophysiology, pathobiology, and emerging clinical perspectives. Journal of the American College of Cardiology. 2017 Apr 4;69(13):1718-34.
3. Garcia-Rivas, G., Jerjes-Sánchez, C., Rodriguez, D. et al. A systematic review of genetic mutations in pulmonary arterial hypertension. BMC Med Genet 18, 82 (2017). https://doi.org/10.1186/s12881-017-0440-5
4. Puk O, Nowacka A, Smulewicz K, Mocna K, Bursiewicz W, Kęsy N, Kwiecień J, Wiciński M. Pulmonary artery targeted therapy in treatment of COVID-19 related ARDS. Literature review. Biomed Pharmacother. 2022 Feb;146:112592. doi: 10.1016/j.biopha.2021.112592. Epub 2021 Dec 25. PMID: 35062063; PMCID: PMC8709827.
5. Humbert M, McLaughlin V, Gibbs JS, Gomberg-Maitland M, Hoeper MM, Preston IR, Souza R, Waxman A, EscribanoSubias P, Feldman J, Meyer G. Sotatercept for the treatment of pulmonary arterial hypertension. New England Journal of Medicine. 2021 Apr 1;384(13):1204-15.