Biopharmaceutical manufacturing processes like cell culture, harvesting and downstream purification are rapidly gaining importance as process robustness and assurance of product safety in these processes are being considered seriously. This article looks into the lean concepts and ready-to-use technologies that are gaining prominence in the manufacturing of biopharmaceuticals.
B iopharmaceuticals have enjoyed good success since the introduction of the first genetically engineered insulin by Eli Lilly in 1982, and we have seen hormones, vaccines, interferons, and the recent triumphs of humanised monoclonal antibodies (Mabs) adding to a long list of commercialisations. As traditional pharmaceutical companies continue their struggle to discover and bring new chemical entities to market, battling with side effects and long development times, those pursuing biopharmaceuticals have found high success rates for difficult healthcare problems whilst meeting a different set of challenges including risks of viral contamination, impurities and potential immune reactions.
Today, the number of "biosimilars" in the biopharmaceutical pipelines has been increasing, especially when considering the industry from a global perspective. In Europe, growth hormone and erythropoietin have been approved in this category, whereas in the US the regulatory approach to "follow-on biologicals" has not been finalised. Although biopharmaceuticals are attractive for many reasons, cutting corners in their development and production is not advisable. The processes of genetic manipulation of host cells, cell culture, harvesting and downstream purification (DSP) are not trivial, and assurance of product safety must be taken seriously.
Manufacturers of biosimilars should not be looking to use different and cheaper manufacturing methods and less analytical rigour than the original developers of protein drugs. Process robustness and safety requirements for biosimilars will always remain the same as for the original proteins. In fact, biosimilar manufacturers will be under greater scrutiny to demonstrate comparability and they may find it easier to use similar processes they have used in manufacturing the originals.
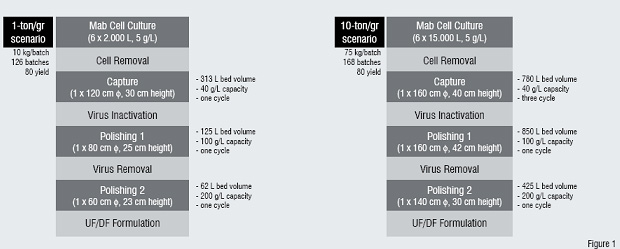
Figure1: DSP scenarios to produce 1 ton and 10 ton of Mab / year.
Output from six bioreactors is handled by one DSP train
We saw how a related area, plasma proteins, was hit by virus contamination in the 1980s, and it is clear that complex biological processes and large, difficult-to-characterise biological molecules are not amenable to the short-cuts available to the producer of small-molecule chemicals. However, technologies are advancing and it is time to take stock of the issues and the direction for improvements.
In Mab production, there is frequent talk of a bottleneck in DSP. This is largely due to the considerable investments that have been made in mammalian cell culture capacity for highly successful products used for rheumatoid arthritis and cancer treatment. Coinciding with the establishment of numerous "six-packs" of bioreactors exceeding 10,000 litres in volume, upstream technologies have improved to the extent that Mab titres frequently exceed 1 g/L. This is a hundred-fold increase over the last 15 years and obviously places significant stress on the capacity of DSP processes that were designed to handle the initial output levels envisaged when the plants were conceived. However, using modern chromatography media in platform processes, it is certainly possible to purify Mabs at levels of around 100 kg per bioreactor batch and 10,000 kg per year. Typically, six bioreactors are used to feed one DSP train, the high titre of the bioreactor being balanced by the short processing time of DSP.
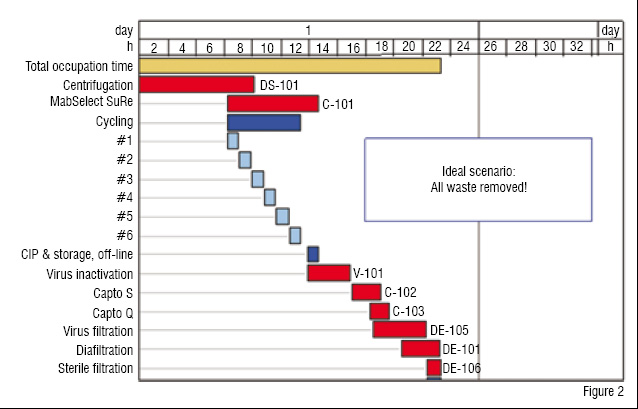
Figure 2: By applying LEAN principles, even a
three-chromatographic-step DSP process can be run in one full day
A process improvement methodology like Lean can be used to find ways to create better flow in a process, take out stops, and reduce non-productive activities such as change-over time between production campaigns, cleaning procedures, or preparation of equipment and process buffers.
In Lean terminology these activities are referred to as "muda" (Japanese for "waste"). While "muda" can be removed easily, some waste is necessary for the technology in use and also useful for flexibility and robustness. Although the output of Mab from each bioreactor has reached impressive levels, it can only be harvested after 10 days (usually it takes even longer to achieve the very high titres frequently reported at conferences), whereas a DSP process can turnaround in two days. By applying Lean, this can be squeezed into 24 hours and with the latest DSP platform approaches, involving only two highly-developed chromatography steps, it should be possible to reduce this even further.
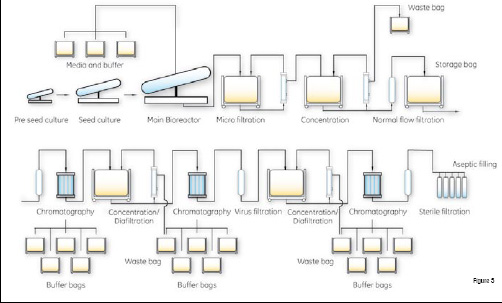
Figure 3: For Mab production platforms,
ready-to-process technology solutions are available for
all unit operations from the bioreactor to final product
The big question is what capacity will be required in the future. The real need is agility, especially for start-up companies and companies entering into the biopharmaceutical arena. There will be few blockbusters that need 10,000 Kg/year as described above. It will be difficult to predict the quantities that will be required for a particular product. Many products in the pipeline are for the same indication or are strict biosimilars aimed at exactly the same patient group. Furthermore, there are developments aimed at increasing the potency or half-life of biopharmaceuticals. Talk of personalised medicine continues with the logical consequence that drugs will be targeted at smaller patient sub-groups, leading to so-called mini-busters. In the race to market, speed is needed to produce small quantities for clinical trials long before investing in large-scale capacity. All of these factors are pushing the required scale of operation down rather than up and increasing the need for flexibility and speed.
Let us consider what is meant by speed. Apart from the classic desire to be first on the market, there are some new angles. One is illustrated in the area of vaccines, where efforts to be prepared for influenza epidemics require shorter timelines than those available with traditional approaches.
A reasonable target is to be able to deliver vaccines within three months from strain identification instead of the current six to nine months with egg-based technology. A promising approach is to use virus-like particles (VLPs). Novavax, a US-based company led by Rahul Singhvi, is developing such an approach based on production methods in insect cells combined with GE Healthcare's ReadyToProcessT production lines. Using a genetically engineered baculovirus vector and insect cell culture in bioreactors, influenza, vaccine can be produced based on VLPs much more rapidly than in traditional egg-based methods.
Today's pressures are pushing the vaccine industry towards greater speed and flexibility, which is enabled by cell culture in disposable bags, rapidly changeable fluid lines, ready-to-use disposable filter cartridges, and the latest innovation, ready-to-process pre-packed production columns for chromatography. By combining these technologies, Novavax believes that it can cut project times in half and start-up costs down to a third (based on calculations at the 100 million dose level). In addition, operator exposure to potential pathogens is limited.
There has been much talk in the last few years regarding the use of the terms like disposable, single-use and ready-to-use interchangeably, when in fact each refers to systems and products with quite different qualities. Ready-to-use technologies bring "plug and play" options to biopharmaceutical manufacturing; many of these are indeed also single use and disposable, but others can be re-used a number of times before disposal. The key, however, with ready-to-use platforms is not disposability, but speed and flexibility.
In the Mab area, standardisation and flexibility will be required. The winners will be able to run large number of projects in parallel, dropping those that fail and switching to the successful ones. Again, the ready-to-use revolution, the move away from stainless steel towards plastic, is key to success. Again, the issue is not disposability per se, rather it is the speed at which a production change can be made, as well as the ability to work in a closed environment and reduce risks related to manual operations. Cross-contamination ceases to be a major headache. Cleaning steps are removed in line with Lean principles, and many validation problems are reduced. For Mabs, all unit operations from the bioreactor to the pure product can be substituted by ready-to-use plastic components, including bioreactors, normal flow filter assemblies, cross-flow filter assemblies, mixers, fluid handling and two or three chromatography steps.
A quick calculation reveals that a 500 litre operating volume plastic bag bioreactor and a Mab titre of 5 g/L would produce 2.5 kg Mab product per batch. Thus a "six-pack" of such bioreactors would potentially be capable of producing 400 kg per year, well into the range of product need for most Mabs currently on the market. Such a setup would potentially allow extreme flexibility, use little space, need low upfront investment, and offer quick commissioning and short installation time compared with classic stainless steel production bioreactors. Cleaning and sterilization steps (typical "muda") in the upstream process would be removed along with their validation, adding more available production time for the facility.
Looking at the DSP of Mabs, with pre-packed, pre-tested and pre-sanitized columns, resin slurry preparation and column packing is not necessary and the corresponding "muda" or waste in the process is removed upfront. Since the devices are stable to typical CIP regimes, it is left on to the user to decide whether or not multiple-use is the preferred and more economical option. The novel pre-packed columns are available in sizes which allow up to about 600 g of Mab to be captured per cycle. In practice this means that the 2.5 Kg of Mab from the bioreactor mentioned above could be captured in a five-cycle operation, followed by smaller columns used in one cycle for final polishing by ion exchange. Savings on the setup side are considerable, in time, in water for injection (needed for the cleaning solutions) and in hardware costs. Since none of these ReadyToProcess solutions require start-up cleaning, the burden of validation studies is also considerably reduced. And finally, it might be argued that a ReadyToProcess solution requires a smaller staff of highly skilled operators than does a conventional bioreactor and DSP chain.
This is not to say that the large, dedicated stainless steel plants will not have a place in the future of biopharmaceutical production. They always have an economic advantage in well-established large-scale production centres where there is limited need for change-over and flexibility. There are size limitations on disposable and ready-to-use solutions. Any true blockbuster of the future will still demand hard-piped facilities due to the scale limitations caused by lack of mechanical stability of disposable plastics. A successful biopharmaceutical company will use both approaches, to be agile in the race to market and cost-efficient when the race is won.
At present, the biopharmaceutical industry is undergoing a rapid change. We have not had a major set back, and Mabs in particular hold great promise, as do new approaches to vaccines and certain oligonucleotide-based therapies. With the increasing globalisation of the industry, the new entrants from Asian countries will be able to bypass the old-fashioned inflexible facility setups and leap onto the fast and flexible approaches now available. Now Asian players can also address diseases prevalent in the region and develop innovative new biopharmaceuticals and vaccines.
Although, there are shortages of experienced personnel in the region, this is changing now. Also, there are many uncertainties that producers face before establishing a product in the market. Agility is the key quality that will differentiate the winners. The use of disposables and ready-to-use systems is an important change that needs to be embraced by engineers who traditionally love their stainless steel. This is not because of disposability, but because of the speed and flexibility that they offer, allowing the time saved to be more usefully spent on innovation and re-inventing the industry.
References:
1. Biopharmaceutical benchmarks 2006. Nature Biotechnology 24 (2006) 769 -776 Walsh, G.
2. Process chromatography: Five decades of innovation. Supplement to Biopharm International, Fe. 2007. Curling, J.M.
3. Handbook of Process Chromatography. Development, Manufacturing, Validation and Economics. Second Edition. Academic Press. 2008. Hagel, L., Jagschies, G., Sofer, G.
4. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 25 (19) (2007) 3871-3878. Bright, R.A., Carter, D.M., Daniluk, S., Toapanta, F.R., Ahmad, A., Gabrilov, V., Massare, M., Pushko, P., Mytle, N., Rowe, T., Smith, G., Ross, T.M.