The Residence Time Distribution (RTD) is a fundamental chemical engineering concept. By definition, it is the probability distribution of time that solid or luid materials stay inside one or more unit operations in a continuous flow system. It can be used to characterise the mixing and flow behaviour of material within a unit operation. Currently, the pharmaceutical companies are going through a paradigm shift from conventional batch to Continuous Manufacturing (CM). RTD has been recognised as one of the most important tool that have several novel applications in continuous pharmaceutical manufacturing.
The Residence Time Distributions (RTD) can be determined experimentally through the injection of a tracer material into the process in the form of a pulse or a step. To achieve this, the outlet tracer concentration is measured until the effects of the injection settle down. A key aspect to be considered during the experimental determination of residence time distributions is the tracer selection. The addition of tracer should not influence the flow properties of the bulk powder while still being readily detectable through analytical techniques or any other methods. The RTD determination and its modelling is very useful for continuous pharmaceutical manufacturing.
In this article, the development of RTD model and its applications for continuous pharmaceutical manufacturing process have been highlight. The real time product quality assurance and material traceability are some of main applications of RTD model, widely spreading across pharmaceutical industries. The RTD based control system, ensures the drug concentration in final tablets while the material traceability framework tracks the identity of batch and lots of the product produced. The materials need to be traced for regulatory perspectives and to recall a specific batch of the products if needed. RTD model has been also used to characterised the unit operations involved in CM as well as the integrated line.
1. Process Description
A continuous direct compaction tablet manufacturing pilot plant has been developed, situated at ERC-SOPS, Rutgers University. The snapshot of the pilot plant is previously reported. The pilot plant is built in three levels at different heights to take advantage of gravitational material flow. The top level is used for feeder placement and powder storage, the middle level is used for delumping and blending, and the bottom level is used for compaction. There are three gravimetric feeders (K-Tron)-with the capability of adding more- that feed the various formulation components (API, excipient, lubricant etc.). A co-mill (Glatt) is also integrated after the feeder hopper primarily for de-lumping the powders and creating contact between components. These feed streams are then connected to a continuous blender (Glatt) within which a homogeneous powder mixture of all the ingredients is generated. The chute is placed in between blender and tablet press. The chute has interface to integrate the sensors. Finally, the outlet from the blender is fed to the tablet press via feed frame. The process flow sheet is previously reported.
2. Residence Time Distribution Model
There are two approaches commonly used for RTD modelling as discussed in following sections.
The tank-in-series model approximates the RTD of a system as a series of equally sized CSTRs, resulting in a realistic mixing description. The number of tanks is an integer varying from 1 to infinity, and a larger number of tank results in a narrower RTD, tending to a Plug Flow Reactor with no axial mixing (PFR) as the number of tanks tends to infinity. The tank in series model is represented by following mathematical equation:
Where ?, is the mean residence time and n is the number of Continuous Stirred Tank Reactors (CSTRs). E(t) is the residence time distribution. TIS means tank in series and PFR means plug flow reactor. The experimental RTD data can be used to fit into this equation by determining the number of tanks and mean residence time.
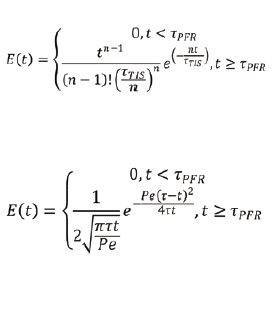
The axial dispersion model describes the RTD of the system as an ideal plug flow within a tube superimposed by a diffusion term resulting in a system characterised by back mixing. The dispersion RTD function for open-open boundaries is given below (Taylor, 1953).
where ? is the mean residence time, ?PFR is the system dead time, and Pe is the Peclet number. As the Peclet number tends to infinity, the behavior of the system approaches an ideal plug flow reactor, where no axial dispersion is present. This model has a similar fitting procedure as the tank in series model with the only difference being the fact that the main fitted parameter of the dispersion model is the Peclet number (Pe), which represents the ratio between convective and diffuse transport.
There could be several applications of the RTD model in continuous pharmaceutical manufacturing process. Some applications are highlighted in following sections.
The RTD model can be used to characterised the unit operations involved in continuous pharmaceutical manufacturing plant. Specifically, it is very useful tool to understand the mixing inside a unit operation. For example, the RTD model can help to understand the mixing inside a feeder and thereby supports to establish the efficient feeder refill strategy. Similarly, the RTD model is useful to understand the mixing capacity of a blender and thereby to improve its performance. Wider RTD means more mixing. The blender operating condition and blade configuration can be adjusted to improve the mixing if needed. The mixing n continuous pharmaceutical manufacturing is happening till the powder/granules goes to the dies. Therefore, the RTD model can also help to understand the mixing in feed frame and thereby the final uniformity of the blends/granules entering to the die.
The RTD model of integrated continuous pharmaceutical manufacturing process is extremely important to characterise the line. It provides mean residence time of the powder particles entering the line. The overall mixing and segregation happening in the line can be also characterised by employing the RTD experiments and corresponding model. RTD has been recognised as one of the important tool to model the intergraded continuous pharmaceutical manufacturing process.
The RTD mode can predicts the drug concertation. So it can be used as an alternative measurement of drug concertation wherever the actual sensors are either not available for real time monitoring or cannot be integrated because of ‘sensor integration constraints’. Following are some of the possible scenarios where RTD model is useful:
If the API (Active pharmaceutical ingredient) concentration in the formulation is too low, then it cannot be accurately detected by PAT (process analytical technology) sensor in real time. However, there could be offline sensors to measure this variable. In that scenario, the RTD model can be used to predict the drug concertation. The predicted drug concertation can be used for real time monitoring, process control or diversion of out of spec products.
For continuous pharmaceutical manufacturing via wet granulation, the drug concertation need to be measured and assured at the outlet of wet granulator. Ifthe drug concertation in granule cannot be measured in real time (may be because of low concentration), then RTD model can be used to predict it.
In case of continuous pharmaceutical manufacturing vid direct compaction, the RTD model can be used to predict the drug concentration at blender outlet, feed frame outlet and of final tablets. The real time monitoring technique of drug concentration of final tablet is currently not available and therefore, RTD model can play an important role here. The tablet potency is normally measured offline for example using Bruker MPA.
The integration of Process Analytical Technology (PAT) sensor into feed frame for real time monitoring of drug concertation is still a challenging task. Therefore, RTD model can be used to predict the concentration of the drug just before entering to the die.
The real time measurement of the control variable is an essential requirement for feedback control implementation. As discussed in previous section, the RTD model can be used to predict the drug concentration and thereby it can enable the real time feedback control. Any control algorithm such as classical Proportional Integral Derivative (PID) or advanced Model Predictive Control (MPC) can be used for feedback control. One example, of RTD based feedback control is the continuous granulation process where drug concentration is too low to be detected by PAT. In this case, using the RTD model of granulator, the drug concertation at granulator outlet can be predicted. This signal then can be used to manipulate the upstream dilution feeder flow rate in order to get the consistent drug concentration in granules.
The real time prediction of the drug concentration using RTD model can also enable the feed forward control. The feed forward controller takes the proactive actions and therefore it can mitigate the effects of disturbances before it will propagate to the final product. Thereby, the feed forward controller can reduce the wastage of the final product significantly. The feed forward controller is essentially a mathematical model derived in a very specific way that takes the measured disturbance as the input and generate the corrective actions proactively in real time. In this case, the predicted drug concentration using RTD model will be used as the input disturbance for the controller. One example of RTD-based feed forward controller in continuous pharmaceutical manufacturing is discussed here. The RTD-based feed forward controller can be used to assure the desired tablet potency. The drug concertation of powder blend measured by NIR is the input for this feed forward controller. The feed forward controller then manipulates the fill depth to keep the tablet potency at consistent level. Meaning that, if the drug concentration in blend detected by NIR is higher/ lower than desired value then the feed forward controller will reduce/increase the size of the tablet so that the total drug contents in the tablet is same. However, the change in tablet size should be within the permitted limits by regulator. There should be one additional controller to maintain the consistent hardness. This controller manipulates the tablet thickness to keep the tablet hardens at consistent level.
The continuous pharmaceutical tablet manufacturing process via Wet Granulation (WG) involves dryer and some hoppers (among other unit operations) which are not truly continuous and have larger dead time. Therefore, for continuous WG process, out of spec granules need to be diverted in order to avoid mixing bad granules with good granules. The RTD model can be used to divert the out of spec granules in real time. A PAT sensor can be used for real time monitoring of drug concentration of the blend entering the granulator. This creates a real time availability of the inlet drug concentration data at the entry of the granulator. The diversion strategy then uses this inlet concentration to determine a signal for the diversion strategy that can accurately be used to reject granules that are out of tolerance limits at the outlet of the tablet press. The RTD is used to predict the outlet concentration from the inlet concentration. The predicted signal is then used to initiate the diversion.
A drug concentration based diversion system is an intrinsic requirement for continuous pharmaceutical manufacturing. For the continuous manufacturing process, an upstream disturbance could propagate downstream if it has not been controlled locally or if the local control is not efficient causing overshoots. Depending on the performance of downstream unit operations, this disturbance could amplify or diminish. Nonetheless, due to this disturbance propagation, there is a need to control or be able to mitigate situations that have the capacity to deteriorate end product quality. The drug concertation can be measured before tablet press using PAT. This creates a real time availability of the inlet drug concentration data at the entry of the tablet press. The diversion strategy then uses this inlet concentration to determine a signal for the diversion strategy that can accurately be used to reject tablets that are out of tolerance limits at the outlet of the tablet press. The RTD is used to predict the outlet concentration from the inlet concentration. The predicted signal is then used to initiate the diversion.
One area that is highly desired to be systematically investigated is material traceability in continuous manufacturing systems. The Residence Time Distribution (RTD) method can be used for material traceability in continuous pharmaceutical tablet manufacturing process. By conducting tracer experiments using a pulse or step change of detectable material at the inlet, the response of the tracer at the outlet can be measured. The amount of time it takes for tracer to first be detected at the outlet, as well as the time it takes for clearance of the tracer material provide valuable information for material traceability. Utilising the minimum and maximum residence times for the continuous line pre-production, raw material batch changes that occur during feeder refill can be traced at the outlet of the process. In the case that one component of the formulation changes batch number, theoretically, the tablet quality should not change given that the process conditions are unchanged. However, for material traceability purposes, the tablets containing raw material from one batch and another need to be distinguished. This raw material batch change occurs during a single manufacturing order and with no changes to the process conditions, therefore is still the same batch. However, we can assign these tablets containing new raw material batch to a separate ‘lot’, within the current tablet batch. This would be a ‘specific identified portion of a batch’ in which the tablets contain material from a raw material batch different from the previous lot. The idea is that, when tablets are released, with a specific batch number and lot number, it exactly traces to what raw material batches may be present in the tablet. By changing the lot based on raw material batch composition in the tablets, it can be certain, if recall was required for specific raw material batch, which lot of tablets must be recalled as well, without recalling the entire batch, many of which tablets contain none of the recalled raw material batch.
The Residence Time Distribution (RTD) model is an important tool that has several applications in continuous pharmaceutical tablet manufacturing. In this article, some of those applications have been highlighted. The RTD has been extensively used for the characterisation of unit operations performance involved in continuous pharmaceutical manufacturing as well as the characterisation of whole line. The RTD model has potential to use for the prediction of drug concentration where the conventional sensing method is either not available or cannot be integrated with the plant. Therefore, the RTD model can be also use for feedback and/or feed forward control. Interestingly, the RTD model has been recognised as the essential tool to enable the diversion of out of spec intermediate granules and/or final tablets. The material traceability as required by regulator and essential for product re-call can be also achieved using RTD model. The RTD model enable the real time assurance of drug concentration and material traceability in continuous pharmaceutical manufacturing which is a significant advancement in pharmaceutical industry and is considered essential for real time release as well as patient safety.
Acknowledgements
This work is supported by the US Food and Drug Administration (FDA), through grant 5U01FD005535, and National Science Foundation Engineering Research Center on Structured Organic Particulate Systems, through Grant NSF-ECC 0540855.