Respirable Engineered Spray Dried Dry Powder represents a technology to generate a low bulk density dry powder that can be inhaled via slow and deep inhalation using a simple to use, low cost Dry Powder Inhaler such as the Plastiape RS00 Mod 2 Low Resistance Device for therapeutics delivered both regionally to the lungs as well as to the systemic vasculature using the lungs as a portal. The particles are entirely homogeneous in composition and allow the pharmaceutical actives to be stabilised in an amorphous glassy state which results in stability at ambient conditions. Case Studies for Budesonide, Leuprolide and Tobramycin Sulfate are discussed along with the Regulatory Strategy along the 505(b) 505(b)(2) regulatory route pathway..

The dichotomous branching of the lungs from the 0th generation (the trachea) to the 23rd generation of the lungs offers a surface area of 110m2 of the alveolar region of the 23rd generation where it takes a red blood cell approximately 0.25 seconds to traverse the entire network where gas exchange occurs and the RBC actually travel in single file. This deep lung membrane provides a half life of delivery for a hydrophobic molecule of <30 seconds and allows hydrophilic molecules also to penetrate to the systemic vasculature hence allowing the lungs to be used either for regional delivery or as an entryway to the systemic vasculature depending on the disease being treated and provides an efficient bypass to the “first pass” effect through the liver as well as access to the central nervous system.
The bulk density of spray dried powders may be engineered to be 0.6-0.8g/cc or 0.06-0.08g/cc which allows delivery to the local lung area (mid lungs) for the treatment of diseases such as asthma or infectious diseases versus using the ultra-low density bulk powder for delivery to the deep lungs and thence to the systemic vasculature.
When slowly and deeply inhaled (inhalation flow rate of 25-35LPM), the particles of homogeneous composition are able to traverse the bend in the throat and upon reaching the 3rd generation of the lungs, gravitational sedimentation takes over (where the Reynolds number of the flow is ~0) to bring the particles to the deep lung as a natural consequence.
One of the most simple devices (a thermal aerosol vapor inhaler) using a coated API without any excipients using an airflow trigger (at 20 LPM) to actuate the burning of a fuel coated inside a steel box housed in a polycarbonate housing allows a hydrophobic API such as Loxapine to be inhaled directly through the lungs to the systemic vasculature with a Tmax of 2 minutes.
Similarly, tobramycin sulfate which was used as a treatment for pseudomonas aeruginosa infection in the lungs of cystic fibrosis patients resulted in the patients sitting in front of a table-top Nebulizer inhaling a 300mg dose over a period of 30 minutes with approximately 9% of the dose delivered to the whole lung. A new delivery method using the ultra-low density spray dried dry powder resulted in a reduction of the dose to 112mg taken over 5 minutes with an active content of 28mg tobramycin in each of 4 capsules (50mg bulk powder fill, inserted into a DPI, pierced and orally inhaled).
Details around Regulatory Strategy of the 505(b) 505(b)(2) regulatory route pathway in the US or also known as a Hybrid Application in Europe have already been discussed previously in an invited paper including the clear differences shown by gamma scintigraphy studies of whole lung deposition comparing the Pulmicort pMDI, Pulmicort Turbuhaler and Inhale Therapeutic Systems first generation spray dried calcitonin powder.
1. Leuprolide
Luteinizing hormone releasing hormone (LH-RH) agonists are currently used for treatment of disease states susceptible to excessive or detrimental concentrations of steroid sex hormones, such as endometriosis or prostate cancer. Dosing is limited to parenteral or nasal administration due to the low oral bioavailability of LH-RH agonists, which is secondary to extensive first pass metabolism. The major products, such as Zoladex® (AstraZeneca Pharmaceuticals LP, Wilmington, DE) and Lupron (TAP Pharmaceutical Products Inc., Lake Forest, IL) are available as depot parenteral formulations that require dosing once every one to three months, depending on the formulation and indication. The use of monthly depot products greatly increases patient convenience and compliance and reduces the frequency of painful injections, but the irretrievable and long-term nature of these formulations limits the physician’s ability to adjust dosing to manage adverse events. Product labelling indicates that chemical castration results in most patients receiving the recommended dose, leading to a significant incidence of vasomotor symptoms (hot flashes), headache, and to a six-month limit of therapy secondary to clinically significant loss of bone mineral density.
In the case of LH-RH agonists, pulmonary delivery offers a stable, reproducible drug input profile similar to that of oral dosing, free from changes in drug input (such as initial drug bursts) which occur with time following administration of depot formulations, and the ability to adjust the dose in response to the clinical situation, or stop therapy if indicated or desired by the patient. Thus pulmonary delivery of leuprolide is expected to improve patient care by providing reversible, adjustable, efficacious treatment of endometriosis using a proven LH-RH agonist, but using a dosing procedure in the patient’s home that is little more complex than oral administration.
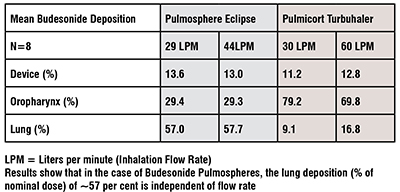
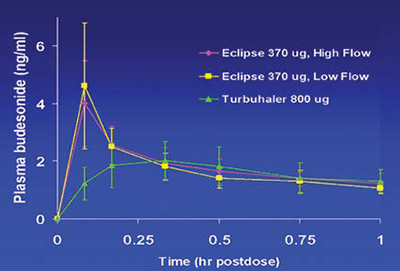
The PulmoSphere® particle engineering technology produces ultralow density powders for inhalation using portable, passive dry powder inhalers, without the need for chlorofluorocarbon propellants. Proof-of-concept clinical studies have been completed with both the corticosteroid budesonide and the anti-infective tobramycin sulfate. In the budesonide study, 67 per cent% of the emitted dose was deposited in subject’s lungs independent of peak inspiratory flow rate, while the tobramycin clinical study illustrated that powder doses as large as 25mg could be delivered efficiently in a single inhalation.
1, 0.5x Oropharyngeal Deposition
2, 2.1x Lung Deposition from ~1/2 the dose
Fivemg of the leuprolide PulmoSphere powder (0.75mg leuprolide acetate) was hand-filled into size #2 hydroxypropylmethylcellulose (HPMC) capsules (Shionogi, Nara, Japan). The capsules were then loaded into the Turbospin® dry powder inhaler (PH&T, Milan, Italy) for aerosol administration to subjects. The Turbospin (now referred to as the Inhale T-326 dry powder inhaler) is a portable passive dry powder with a device resistance of 0.09 (cm H2O1/2)/(L·min-1). Leuprolide acetate for injection (Lupron®, TAP Pharmaceutical Products Inc., Lake Forest, IL) was supplied in vials containing 2.8ml of leuprolide acetate (5mg/ml). The formulation also includes sodium chloride for tonicity adjustment, and 9mg/ml of benzyl alcohol (preservative). The leuprolide concentration was diluted to 0.71mg/ml with normal saline prior to injection of a 1 ml bolus.
2. Budesonide
A platform technology comprising a respirable engineered spray dried dry powder demonstrates flexibility and diversity in the development of a flow rate independent whole lung drug delivery of a homogeneous, low bulk density and room temperature stable product that can incorporate up to 3 APIs (e.g. ICS, LABA and LAMA of Astra Zeneca’s Breztri based on the Pulmosphere Platform-Pearl Therapeutics a spinoff of Inhale Therapeutics who were licensed to use the Pulmosphere Technology in pMDIs was bought by Astra Zeneca). This results in a low cost, self administered medication and is amenable to a large variety of APIs from small molecules, peptides, globular proteins, monoclonal antibodies, siRNA, mRNA, DNA and whole killed vaccines all of which are stabilised in an amorphous glassy state when spray dried due to the fact that the water evaporation from the drying droplet in <300ms, results in a Joule Thomson cooling effect that is instantaneous. The 505(b)(2) regulatory route offers a rapid entry to market-typically a Phase 1 for Safety followed by a Pivotal Phase 3 for both Intranasal as well as Pulmonary Delivery.
REFERENCES
1. Patton, JS: Mechanisms of macromolecular absorption by the lungs, Adv Drug Deliv Rev 1996; 19:3-36
2. Schanker, LS: Drug absorption from the lung, Biochem Pharmacol 1978; 27:381-385.
3. Berardis, D, Fornaro, M, Orsolini, L, Iasevoli, F, Tomasetti, C, Bartolomeis, A, Sevaroni, N, Valchera, A, Carano, A, Vellante, F, Marini, S, Piersanti, M, Perna, G, Martinotti, G, Giannantonio, M: The Role of Inhaled Loxapine in the Treatment of Acute Agitation in Patients with Psychiatric Disorders: A Clinical Review, Int. J. Mol. Sci. 2017; 18:349-364
4. Geller, DE, Weers, J, Heuerding, S: Development of an Inhaled Dry Powder formulation of Tobramycin using Pulmosphere™ Technology. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2011; 24(4): 175-182
5. Masters, K: Spray drying-an introduction to principles, operational practice and applications. 1972; London: Leonard Hill Books
6. Platz, RM, Patton, JS, Foster, L, Eljamal, M: Methods of spray-drying a drug and a hydrophobic amino acid; 2002; USA Patent: 6,372,258
7. Tsapis, N., Bennett, D., Jackson, B, Weitz, DA, Edwards, DA.: Trojan particles: Large porous carriers of nanoparticles for drug delivery. 2002; Proceedings of the National Academy of Sciences of the United States of America, 99:12001–12005.
8. Raula, J, Eerikäinen, H, Kauppinen, EI: Influence of the solvent composition on the aerosol synthesis of pharmaceutical polymer nanoparticles. 2004; International Journal of Pharmaceutics, 284:13–21.
9. Elversson, JE, Millqvist-Fureby, A: Particle size and density in spray drying-effects of carbohydrate properties. 2005; Journal of Pharmaceutical Sciences, 94:2049–2060.
10. Fäldt, P, Bergenståhl, B: The surface composition of spray-dried protein–lactose powders. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1994; 90:183–190
11. Duddu SP, Sisk SA, Walter YH, Tarara TE, Trimble KR, Clark AR, Eldon MA, Elton RC, Pickford M, Hirst PH, Newman SP, and Weers JG: Improved lung delivery from a passive dry powder inhaler using an engineered Pulmo-Sphere powder. Pharm Res. 2002;19:689–695.
12. Weers JG, Bell J, Chan H-K, Cipolla D, Dunbar C, Hickey AJ, Smith IJ: Pulmonary formulations: what remains to be done? J Aerosol Med Pulm Drug Deliv. 2010;Suppl 2:S5–S23.
13. Newhouse MT, Hirst PH, Duddu SP, Walter YH, Tarara TE, Clark AR, and Weers JG: Inhalation of a dry powder tobramycin pulmosphere formulation in healthy volunteers. Chest. 2003;124:360–366.
14. Borgström L, Olsson B, and Thorsson L: Degree of throat deposition can explain the variability in lung deposition of inhaled drugs. J Aerosol Med. 2006;19:473–483.