Pharmaceutical tablets have been traditionally classified as good (within specifications) or bad (out of specifications) based on offline measurements of representative samples. Several assays based on tablet samples are necessary to ensure product quality and must be satisfied before releasing the tablets into the market. However, there are no methods and tools available that can be used for real time assurance of tablet drug content. In this work, a framework for the implementation of a tablet diversion system based on Residence Time Distribution (RTD) is presented. The proposed diversion system is essential to assure the drug content of the tablets produced via continuous manufacturing. A tablet diversion system is created according to the introduced framework, and the proposed diversion system is implemented in a commercial control platform, where its functionality is demonstrated using the developed RTD model.
Residence Time Distribution (RTD) is a probability distribution function that describes how long a fluid or powder element spends inside a given operation. Currently, the Continuous Manufacturing (CM) is evolving as a preferred platform for pharmaceutical products involving solid dosages forms. Therefore, the pharmaceutical industries are going through a paradigm shift from conventional batch manufacturing to advanced Continuous-Manufacturing (CM). However, the RTD-based diversion system is desired for continuous pharmaceutical manufacturing to assure the drug contents of the tablet to be released to the market.
The objective of this work is to demonstrate the implementation of RTD-based tablet diversion system into a novel continuous direct compaction pharmaceutical tablet manufacturing pilot-plant.
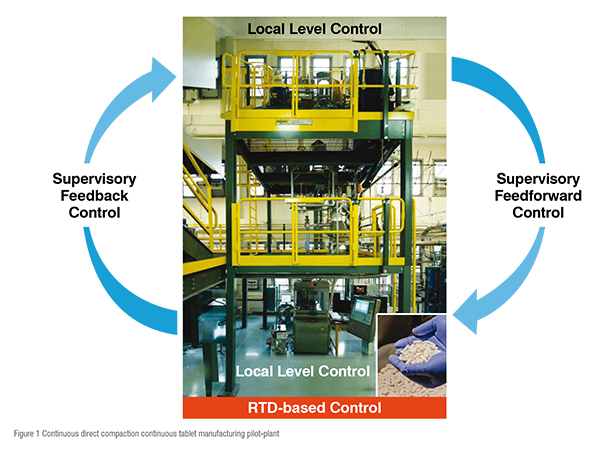
The continuous pharmaceutical manufacturing pilot-plant has been built at C-SOPS, Rutgers University that has been adapted by industries. This process has been extensively studied. The snapshot of the pilot-plant is shown in Figure 1. The process and pilot-plant description has been previously reported.
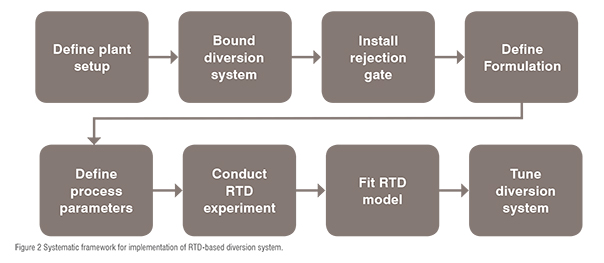
A systematic framework has been developed to guide the implementations of diversion systems based on RTDs (see Figure 2). It is known that the residence time distribution of a system is characteristic of its unit operations. Hence, the plant setup needs to be defined before any RTD determination efforts occur. It is also important to establish the type and location of the PAT sensors. Once the plant configuration is fixed, the boundaries of the rejection system must be defined. The downstream boundary should be chosen based on the closest location, downstream of the unit operation producing undesirable products, where the diversion gate can be installed. The upstream boundary is dependent on the closest PAT sensor upstream of the unit operation.
The installation of the diversion gate and its integration with the control system starts with the definition of the kind of actuation used to operate the gate. Most commercially available systems have either electric or pneumatic actuation. Once the gate is installed, it is necessary to understand any transport delays between the actuation in the control system and the actual actuation in the gate. This delay must later be incorporated in the RTD of the system.
As previously mentioned, RTDs are heavily influence by material flow properties and process parameters. An RTD is only valid for the formulation and processing parameters at which the experiments were conducted, and extrapolations are rarely valid. For this reason, the next two key steps in the framework are fixing the powder formulation to be used and fixing any process parameters that can influence the mixing or the mass flow rate of the system.
The most important step in the implementation of the tablet diversion system is the experimental determination of the system’s RTDto the experimental data and tuning the diversion system. Model fitting is done to the experimental data and tuning the diversion system. Model fitting is done to obtain a clean RTD, without noise, that represents the system. Once the model is obtained and integrated, the tuning of the diversion system occurs. In this step, the safety margin of the RTD prediction is determined. This margin can be tuned by tightening the nominal limits for diversion by a tuning constant.
Three simulated scenarios have been created to demonstrate the implementation of the RTD-based diversion system in a commercially available control platform (Figure 3). The NIR signal has been simulated using an input parameter block and the F(t) coefficients used in this demonstration correspond to the response of a first order system.
In the first scenario (Figure 3a), a pulse with 10 seconds duration and 1 per cent magnitude was applied to the system. The pulse spreads across the tablet press according to the RTD resulting in predicted concentrations that do not trigger the rejection mechanism. This result is a clear example of a situation where an RTD-based diversion yields a better performance than the traditional time delay based diversion system. The second scenario (Figure 3b) consists again of a pulse with 10 seconds duration but with 12 per cent magnitude. The larger magnitude results in a disturbance that violates the concentration limits and triggers the diversion system for approximately 17 seconds.
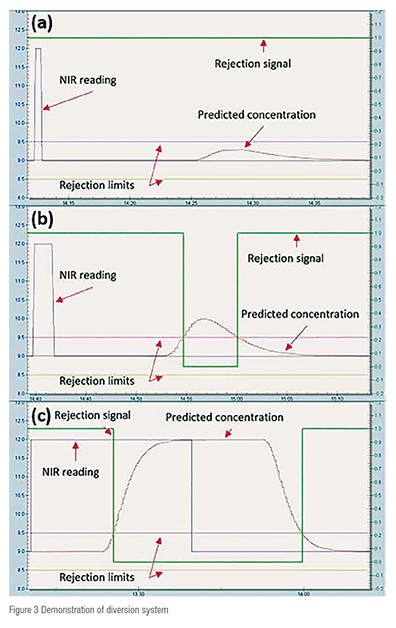
When a step disturbance with a magnitude of 3 per cent is applied to the system (Figure 3c), the diversion system is triggered after two seconds and only returns to its normal state 6 seconds after the input concentration value is brought back to 9 per cent. This scenario replicates a situation where a traditional delay based diversion system would achieve the same performance as an RTD-based system.
It is essential to assure the drug concentration of the tablets before releasing it into the market. A combination of traditional feed forward/feedback control strategy together with a tablet diversion system can be used to achieve this. The RTD-based control system can be used to divert the out of specification tablets produced during continuous pharmaceutical manufacturing. A systematic framework to implement the RTD-based control system has been developed and applied to the continuous pharmaceutical manufacturing process. The proposed systematic framework supports the paradigm shift of pharmaceutical tablet manufacturing from conventional QbT-based batch-wise production to QbD-based continuous production.
This work is supported by the National Science Foundation Engineering Research Center on Structured Organic Particulate Systems, Rutgers Research Council and U.S Food and Drug Administration (FDA).