Safe and effective vaccines to prevent COVID-19 caused by Severe Acute Respiratory Syndrome coronavirus 2 (SARSCoV-2) have been developed within a year after the onset of the pandemic. As of December 2021, 54.5 per cent of the world population had received at least one dose, but Africa is lagging behind. Full, lasting protection against severe disease will require two doses plus a booster, and preferably incorporating emerging virus variants such as omicron.
As a rule of thumb in epidemiology, the world faces a pandemic once every 100 years. The previous one was the Spanish flu in 1918. At the end of World War I, a new strain of the influenza virus, H1N1, broke out in the United States. The disease mainly affected young adults, initially recruits in army camps, who were in training to be sent to Europe. Ultimately, more American soldiers died from the flu than in the battlefields of Europe. During wartime, most news-papers were censored, and coverage of the outbreak of this very serious form of influenza was prohibited. However, Spain was neutral during the First World War, and Spanish newspapers did report on this disease, which subsequently became known as Spanish flu.
The Spanish flu lasted for two years and by the time it was over, more than 50 million people worldwide had died. Every place on earth was affected. Douglas Island, a small off the coast of Alaska, in the second week of November 1918 had 100 patients with influenza admitted to the local hospital. There was one death, Maude Kelly, who was a nurse of the influenza patients at the local hospital.
That after the Spanish flu another pandemic would come was to be expected. The question, therefore, was not “if” but “when”. The risk of outbreaks of diseases that can spread from animals to humans inevitably increases when animals and humans come into close contact with each other, caused for example by habitat degradation, intensive livestock breeding (e.g., Q fever) and the trade of wild animals. Combined with other factors such as increased air traffic, urbanisation (more than 50 per cent of the world's population lives in urban areas) and climate change, the chances of another pandemic were high. In 2018, the World Health Organization (WHO) listed a number of infectious diseases that can pose a serious threat to public health, for most of them no vaccines or effective drugs are available. One of these could be "Disease X" — a future disease that humans had never seen before that would cause a pandemic. We now know that Coronavirus Disease 2019 (COVID-19) is disease X.
Douglas Island in Alaska already had a local newspaper in 1918: Douglas Island News. In the same issue that reported Maude Kelly's death, it also published a list of 11 Do's and Don'ts to get safely through the influenza pandemic. It is striking that almost all these advices are fully applicable at the time of COVID-19. Just a few examples: wear your face mask, do not ignore the advice of specialists because you do not understand them, do not think that an exception can be made for you, do not think that you cannot contract or spread influenza (for influenza read corona), and finally, don't worry!
COVID-19, a word that did not exist in January 2020, has infected more than 260 million people worldwide and killed 5.2 million on December 2, 2021. Also by December 2 2021, a total of 203,479 scientific publications on COVID-19 had been published, with over 2,000 publications in November 2021, a number that reflects how all efforts are being made to better understand the disease, ways to treat it and prevent it by vaccination.
Naturally, also in 1918 clinicians and scientists worked hard to develop an influenza vaccine. Unfortunately, they were not successful and the Spanish flu pandemic only ended when everyone (on the world!) who was sensitive had contracted the disease, and was either cured and subsequently immune, or had died.
The development of mRNA-based and other vaccines to prevent COVID-19 caused by SARS-CoV-2 has taken place within a year. This speed of development has been unrivalled in the pharmaceutical world, and an example of global collaboration between governments, science and industry. All this took place within a time frame of 12 months, calculated from the elucidation of the sequence of this new coronavirus up to and including the completion of phase 3 studies and the start of the first actual vaccinations. In the publications on the results of the phase 3 studies of both the BNT162b2 mRNA vaccine from Pfizer (Comirnaty) and the mRNA-1273 SARS-CoV-2 vaccine from Moderna, the words "efficacy" and "safety" appear prominently in the title.
Pfizer's mRNA vaccine, BNT162b2, has been tested and found to be 95 per cent successful in preventing COVID-19. The Moderna mRNA vaccine, scores comparably high at 94.5 per cent. Crucial for the success of the mRNA vaccines was the incorporation of modified uracil and it is more than deserved that Katalin Karikó received the 2021 Lasker Award for her work in this field. We would wish the Nobel Committee follows next year.
Seven vaccines have obtained the WHO Emergency Use Listing (EUL), which means that the vaccine meets all safety and efficacy requirements and is (also) suitable in low- and middleincome countries. These vaccines include the mRNA vaccines of Pfizer/BioNTech Comirnaty, and the Moderna COVID-19 vaccine (mRNA 1273), the viral vector vaccines SII/COVISHIELD and AstraZeneca/AZD1222 as well as the Janssen/Ad26.COV 2.S vaccine. Furthermore, the Sinopharm COVID-19 vaccine, Sinovac-CoronaVac, and Bharat Biotech BBV152 COVAXIN vaccine (all inactivated virus vaccines) also have EUL status. Another 109 vaccines are being studied in clinical trials under development.
In December 2021, 54.5 per cent of the population of the world has received at least one dose of a COVID-19 vaccine. A total of 8.03 billion vaccine doses have been administered globally. Merriam Webster has declared vaccine as the 2021 word of the year, while for the Oxford English Dictionary this is vax. In Northern- and Latin America, in Europa, and in Asia-Pacific, over 50 per cent of the adult population has received two doses of a COVID-19 vaccine (Figure 1). It must be kept in mind that vaccine uptake can vary per country. Lowest scoring countries in above continents are Guatamala (24 per cent two doses of vaccine), Bosnia and Herzegovina (22 per cent), Kyrgystan (14 per cent) and Papua New Guinea (2.3 per cent). Overall, only 6 per cent of people in low-income countries have received at least one dose.Especially Africa as a continent lags greatly behind the rest of the world. COVAX, the joint initiative of the WHO, Unicef and GAVI, therefore is of crucial importance to facilitate production and equitable access to COVID-19 vaccines, but also tests and treatment. Shortage of vaccines is a major hurdle, but also the required infrastructure and logistics for vaccine delivery (the last mile that a vaccine has to travel from the production facility to the recipient is the most difficult one) and strong vaccine hesitancy and resistance sentiments should be overcome. Figure:1
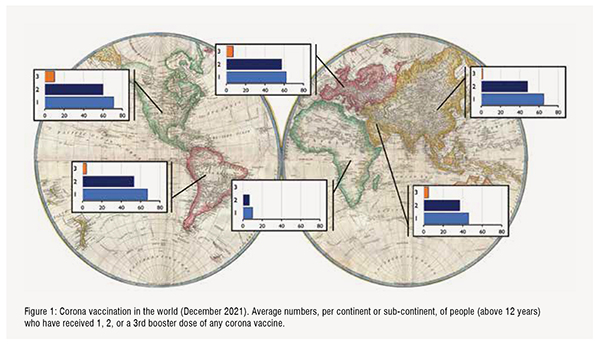
None of the continents, not even a single country, has reached an overall 2-dose vaccination rate to ensure group immunity (with the delta variant estimated to be above 95 per cent). In most publications thus far, two doses of vaccine are indicated as “fully vaccinated”. This term may have to be reconsidered, because booster vaccinations are now implemented in order to protect the most vulnerable groups in the population and in order to reduce spread of the virus variants.
A new SARS-CoV-2 variant of concern has emerged which has been termed termed omicron. Its genome is mutated at 32 positions (of the 671) of the Spike protein, of which 15 are located in the receptor binding domain (122 aa). These mutations have been called a “Frankenstein mix of greatest hits”. At the moment of writing (December 2, 2021) there are still many unknowns: when will the omicron variant displace the delta variant?, will it cause mild or severe disease?, and will the current vaccines offer protection against disease? All major vaccine producers have already started preparing for a next generation of vaccines, which will incorporate (the genetic information of ) these new variants. These vaccines will be needed in 2022 to control SARS-CoV-2 and hopefully put an end to the pandemic.
Selected publications:
Leerboek immunologie. G.T. Rijkers F.G.M. Kroese, Uitgever: Bohn Stafleu van Loghum, Co-auteur: R.H.W.M. Derksen C.G.M. Kallenberg, 2de druk 2016, ISBN 9789036802574
Differences in Antibody Kinetics and Functionality Between Severe and Mild Severe Acute Respiratory Syndrome Coronavirus 2 Infections. Rijkers G, Murk JL, Wintermans B, van Looy B, van den Berge M, Veenemans J, Stohr J, Reusken C, van der Pol P, Reimerink J.
J Infect Dis. 2020 Sep 14;222(8):1265-1269.
More bricks in the wall against SARSCoV-2 infection: involvement of T cells. Rijkers G, Vervenne T, van der Pol P.. Cell Mol Immunol. 2020 Jul;17(7):771-772. doi: 10.1038/s41423-020-0473-0.
Genetics in community-acquired pneumonia. Rijkers GT, Holzer L, Dusselier T.
Curr Opin Pulm Med. 2019 May;25(3):323-329.
Rocking pneumonia. Rijkers GT, Rodriguez Gomez M. Pneumonia (Nathan). 2017 Dec 15;9:18. doi: 10.1186/s41479-017-0043-0.
Dexamethasone and length of hospital stay in patients with communityacquired pneumonia: a randomised, double-blind, placebo-controlled trial. Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, van Velzen-Blad H, Voorn GP, van de Garde EM, Endeman H, Grutters JC, Bos WJ, Biesma DH. Lancet. 2011 Jun 11;377(9782):2023 30.doi: 10.1016/S0140-6736(11)60607-7.