Anti-fungal resistance is increasing, with the global spread of the multi-drug resistant Candida auris since 2008, increasing rates (up to 95 per cent) of azole resistance in Aspergillus fumigatus and the emergence of a new species of Trichophyton in India which is terbinafine resistant. Accurate and rapid diagnosis of most fungal infections is now possible with nonculture based tests, allowing tailoring of both antibacterial and anti-fungal therapy, but culture and susceptibility testing are required to detect resistance. New anti-fungal agents are being developed, but not fast enough to address this problem without minimising empirical anti-fungal therapy in patients and reduction in azole fungicides in crops.
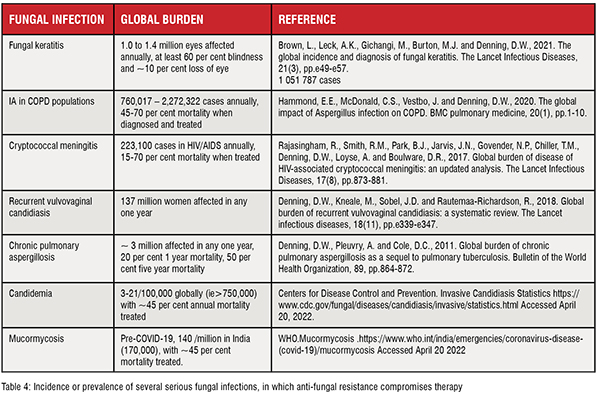
Fungi are ubiquitous in nature and cause disease in humans, animals, and, especially, plants. The range of human fungal infection varies from superficial infections (pityriasis, athlete’s foot, ringworm, nappy rash, and many others), allergic disease (chronic sinusitis and fungal asthma), chronic infections (chronic pulmonary aspergillosis and the NTD mycetoma as examples) to severe life-threatening invasive infections (cryptococcal meningitis, invasive aspergillosis, candidaemia, mucormycosis, etc). Some of these are recurrent infections (vaginal candidiasis) and some fungal infections are contagious. Globally, over 300 million people are threatened by serious fungal infections. Invasive fungal infections are associated with high mortality even with effective anti-fungal management: Cryptococcal meningitis; >50 per cent in the developing world and 15-20 per cent in the USA, invasive aspergillosis;~30 per cent mortality in leukaemia and >90 per cent in liver failure, invasive candidiasis; ~40 per cent mortality even with treatment [GAFFI].
Fungal infections go beyond humans — invading plants, insects, and animals. Fungi infect and destroy food crops reducing food production. It is estimated that one-third of all food crops are destroyed by fungi annually (Fisher et al., 2012). They have a high impact on global nutrition and the economy by affecting the most widely consumedcrops such as rice, wheat, maize, potatoes, and soybeans. The list includes most high-value crops (coffee, bananas, cacao, mangos, and spices). Moreover, food becomes non-edible when fungi produce mycotoxins, commonly in if poorly stored. Fungi are major pathogens of trees, such as chestnut blight, ash dieback, and many others, impacting attempts to ameliorate global warming and incurring major economic loss. More species extinctions are attributable to fungi than any other infectious group, notably many frog species in recent years. Fungal disease leads to decreased biodiversity and indirectly contributes to global warming.
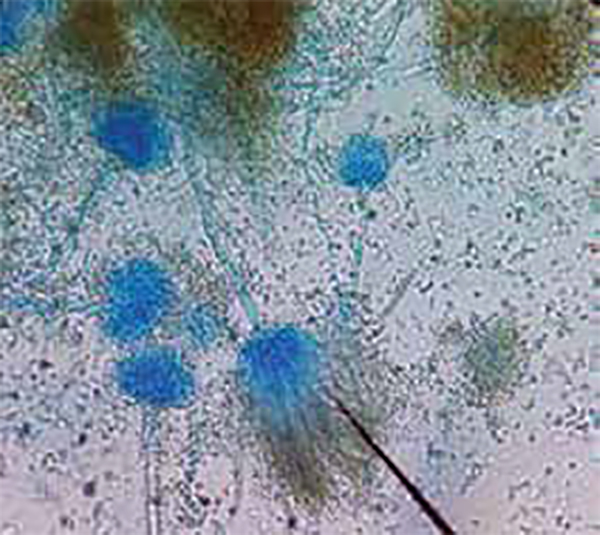
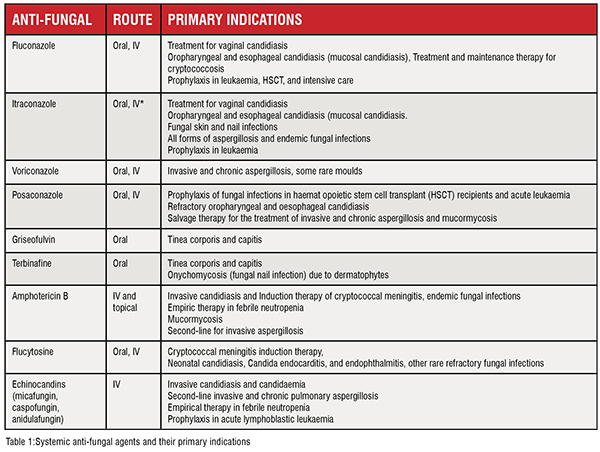
Anti-fungals are used to prevent and treat fungal infections in humans, animals, and plants. Moreover, they play an important role in material preservation and food safety [Verweij, P.E., et al. 2020]. Different classes of anti-fungals act on different targets, inhibiting fungal growth or killing them. A limited number of anti-fungals are currently available for use (Table 1) and new anti-fungals come onto the market at a snail’s pace.
Fungi have large genomes and are very adaptable. Both intrinsic (primary) resistance and secondary (acquired) resistance are well-known. Multiple strategies have been described in different fungi to resist the effect of anti-fungals, including modification of drug-acting sites or voiding the drug out of the fungal cell by efflux as 2 common examples. Many researchers continue to identify new mechanisms of anti-fungal resistance, and in many fungi, several mechanisms coexist in individual cells. Resistance in fungi is both increasing in incidence and increasingly detected. Certain resistant fungi are already widespread across the globe [Denning, D.W., 2022].
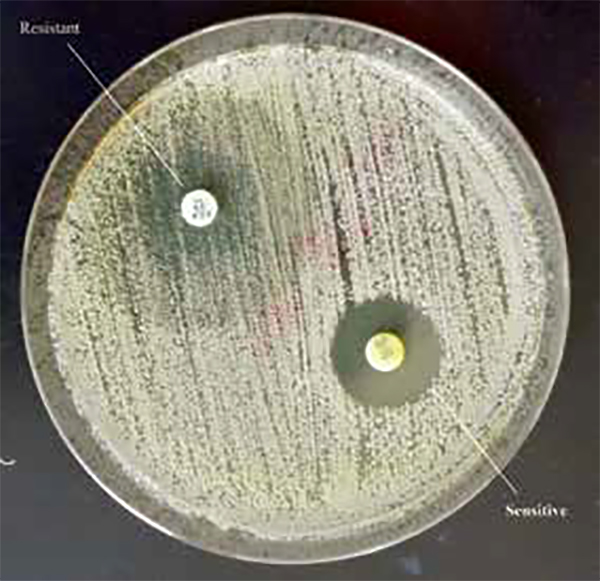
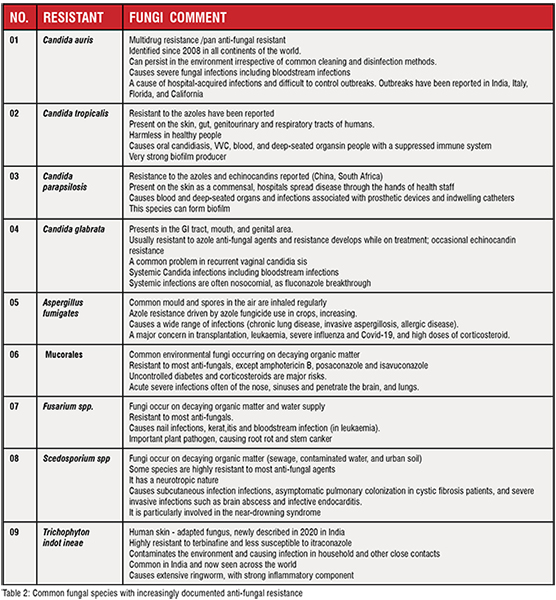
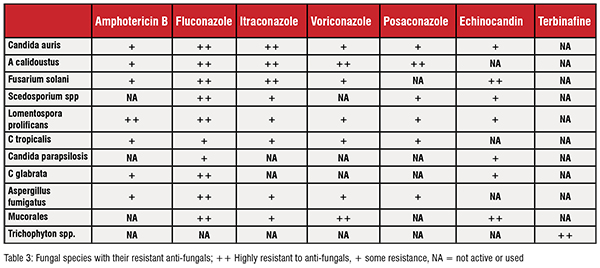
Anti-fungal resistance might be either intrinsic or acquired Naturally, some fungi are not killed by certain anti-fungals, and they are called intrinsically resistant to that anti-fungal. The second group is usually susceptible but alters their susceptibility over time with the presence of anti-fungals and becomes non-responders; Is called acquired resistance. Most of the antifungal resistance is acquired [Denning, D.W., 2022].Interestingly, some of these fungi exhibit resistance to multiple anti-fungals, and some of them are resistant to almost all the available anti-fungals or to a complete class, such as pan-azole resistance. Table 2 shows the most frequently observed resistant fungi, and Table 3 to which anti-fungal fungus is resistant to.
Misuse and overuse of anti-fungals, inadequate infection control in the healthcare sector, poor medical facilities, and lack of awareness are blamed for the emergence and spreading of anti-fungal resistance.
Expansion of preventive and therapeutic use of anti-fungals contributes to the growth of anti-fungal resistance. Repeated drug exposure in the form of prophylaxis or long-term therapy is often associated with the emergence of resistance. When fungi are exposed to suboptimal concentrations, drug-resistant fungal strains are selected. This may be observed among the patients with chronic fungal infections who are supposed to take prolonged anti-fungal treatment courses. Some of these patients show poor compliance with drug regimens (often linked with toxicity or poverty) allowing for the selection of resistance fungi. For example, increased use of azole for prophylaxis and long-term use of azole in chronic conditions are linked with azole resistance in Aspergillus species [Verweij, P.E., et al. 2020].
Anti-fungals have been used widely in the agriculture sector to prevent fungal infections in crops. Azole-based agricultural fungicides are a cornerstone of the crop protection market(including prochloraz, difenoconazole, propiconazole, hexaconazole and tebuconazole) [Darwin, J., et al. 2015]. However, the wide use of fungicides in agriculture is closely linked to creating environmental niches for fungi (Aspergillus in particular) to become resistant. Agricultural fungicides which share similar molecular targets with systemic azole anti-fungals are reported to cause the selection of resistant fungal species. [Verweij, P.E., et al. 2020] [Denning, D.W., 2022].
Anti-fungals play a pivotal role in the treatment of human fungal infections and a successful therapeutic outcome mandates effective anti-fungal treatment. Emerging and spreading antifungal resistance make existing antifungals ineffective. The only oral class of anti-fungals for Aspergillus infections is the azole, and so pan-azole resistance commits patients to intravenous therapy only. Even common fungal infections become harder to treat resulting in severe infections and death. Since a few classes of anti-fungals are available, the emergence of drug resistance severely limits the therapeutic options while multi-drug resistance and pan drug resistance annihilate almost all options. In addition, patients with resistant fungal infections require specific expensive management and prolonged hospital stay.
With the rising at-risk population, the requirement for new strong antifungals is growing. However, the development of new powerful anti-fungals is at a slow pace. Moreover, some anti-fungals show a limited spectrum of activity. Consequently, it is vital to curb anti-fungal resistance because so few anti-fungals are currently available, with only a few new molecules in development.
Moreover, low, and middle-income countries commonly have only some of the quality anti-fungals available in high-income countries. A rising incidence of resistant strains there is not manageable in many of these countries.
The emergence and spread of resistant fungi is now a widespread and global threat. Consequently, we all must fight this problem together, linked with antibacterial resistance countermeasures. A multifaceted approach at the healthcare level, community level, and industry level is necessary.
The health care sectors in each country can contribute to the containment of resistant fungi through meticulous screening by culture, species identification, and susceptibility testing. Good infection control measures are essential, including air protection measures for vulnerable patients. The incorporation of anti-fungal stewardship programs (including rapid diagnostics to minimise unnecessary antibacterial or antifungal therapy) into routine practice will minimise the emergence and spread of resistance among fungi.
Meanwhile, scientists are continuing to develop new laboratory tests to detect resistant fungal infections. Such novel methods will need clinical trials to demonstrate their worth, and these trials need public funding.
Epidemiological research and surveillance are also key to understanding the emergence and spread of anti-fungal resistance. The gathering of data on anti-fungal resistance through anti-fungal resistance surveillance (hospital, and environmental) programs aids in mapping resistance trends. Resistance should be sought not only in the healthcare sector but also in agriculture settings. Surveillance of anti-fungal resistance should be a global enterprise, and databases of anti-fungal resistance openly shared. In addition, financial and technical assistance for low-resource countries should be provided to identify and contain resistant fungal species. The expansion of infrastructure for tracing and testing anti-fungal resistance and for surveillance programs should be a priority.
The consumption of anti-fungals in a responsible manner will help to curtail this issue. As a community, we should avoid sub-therapeutic doses of anti-fungals (and poorly bioavailable generic preparations) which are more likely to select resistant strains. Overthe-counter anti-fungal treatment is strongly associated with the development of anti-fungal resistance in the skin fungus Trichophyton and should be avoided. Azole fungicide use should be minimised and alternative means of addressing crop fungal pathogens utilised.
References
1. Almeida, F., Rodrigues, M.L. and Coelho, C., 2019. The still underestimated problem of fungal diseases worldwide. Frontiers in microbiology, p.214.
2. Darwin, J., 2015. Improving outcomes for patients with fungal infections across the world a road map for the next decade.Gaffi org
3. Denning, D.W., 2022. Antifungal drug resistance: an update. European Journal of Hospital Pharmacy, 29(2), pp.109-112.
4. Duong, T.M.N., Le, T.V., Tran, K.L.H., Nguyen, P.T., Nguyen, B.P.T., Nguyen, T.A., Nguyen, H.L.P., Nguyen, B.N.T., Fisher, M.C., Rhodes, J. and Marks, G., 2021. Azole‐resistant Aspergillus fumigatus is highly prevalent in the environment of Vietnam, with marked variability by land use type. Environmental microbiology.
5. Gaffifungal disease frequency Available at https://gaffi.org/why/fungal-disease-frequency/ Accessed 18/04/2022
6. van Rhijn, N. and Denning, D.W., 2021. Is an azole-resistant Aspergillus hotspot emerging in South-East Asia?. Environmental Microbiology.
7. Verweij, P.E., Lucas, J.A., Arendrup, M.C., Bowyer, P., Brinkmann, A.J., Denning, D.W., Dyer, P.S., Fisher, M.C., Geenen, P.L., Gisi, U. and Hermann, D., 2020. The one health problem of azole resistance in Aspergillus fumigatus: Current insights and future research agenda. Fungal Biology Reviews, 34(4), pp.202-214.