As the number of candidate vaccines approaching human trials increases, vaccine researchers should consider all collaborative research models, as well as look to their colleagues in prevention sciences for collaboration and to learn how to effectively conduct prevention trials.
Over the past decade there has been an explosion in the number of clinical research studies being conducted globally and in particular in resource-limited settings, including in Asia. Researchers and pharmaceutical companies have worked earnestly to develop new treatment, prevention and diagnostic technologies to tackle the global public health challenges of diseases such as HIV, TB, malaria, influenza and new and emerging infectious and non-infectious diseases.
In March 2011, listings of clinical trials registered in the US FDA's clinical trial registration database showed that almost 104,000 clinical trials are currently registered in 174 countries worldwide. Table 1 indicates the breakdown in Asia alone. This will only be a fraction of the actual clinical studies being conducted as those not intended for submission to the US FDA, or receiving financial support from US sources do not need to be registered in this database.
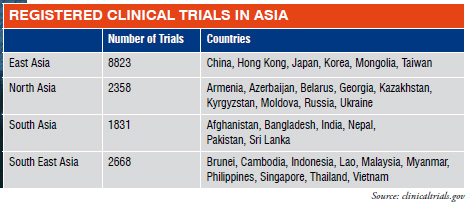
With so many clinical research studies being conducted or planned, there is a growing need to increase the number of sites capable of conducting clinical trials that meet international regulatory and ethical standards, including Good Clinical Practices (GCP). With too few acceptable clinical research sites, those that do exist will be over-burdened and the quality of the clinical research will likely suffer as a result. Staff and investigators may have insufficient time to dedicate to each research protocol, affecting the quality of the results and possibly patient safety as well. In high-burden research sites study findings can also become less relevant to real-life settings as patients become part of the research machine. This can lead to health seeking behaviours and treatment or intervention compliance that differs from ‘normal’ patients and may confound the application of clinical trial results.
To expand the pool of high quality clinical research sites many research funders are establishing collaborative models or networks to build research site capacity. The structure of these networks tends to be similar, including:
This collaborative model brings many different expert groups together to build the capacity of clinical research sites to conduct high quality clinical research. This model has been used by many including the US National Institute of Health (NIH) for their HIV Prevention Trials Network , with Asian sites in China, India, Russia and Thailand, and the South East Asia Infectious Disease Clinical Research Network (SEAICRN) , with sites in Indonesia, Thailand and Vietnam. The US Center for Disease Control (CDC) is also using this model for their TB Clinical Trials Consortium with Asian sites in Hanoi and Hong Kong.
FHI has played a key role in these Networks in multiple capacities including site research capacity building. To establish such Networks requires time and financial resources; but, once established they offer the ability to conduct high quality clinical trials complying with international standards. Data from multi-site and multi-country studies are likely to be more comparable as standard and consistent study systems and procedures have been implemented across all sites. This is evidenced by the number of peer-reviewed publications resulting from the activities of these networks. SEAICRN, for example, reported supporting 95 publications in the period 2006-2010.
A slightly different model is one where research funders seek to build the capacity of young or new research investigators particularly in resource-limited and disease-endemic countries. In such a model, investigators receive training in all aspects of clinical research starting from designing a protocol, through research implementation and reporting. The US NIH adopts this model for their International Clinical Sciences Support Center to provide support to investigators funded by their Department of Microbiology and Infectious Diseases (DMID) and the National Institute of Allergy and Infectious Diseases (NIAID).
As of March 2011, some 1500 investigators have been supported by the ICSSC worldwide, including in Bangladesh, China, Hong Kong, India, Nepal, Papua New Guinea, Philippines, South Korea, Sri Lanka, Taiwan, Thailand, and Vietnam.
Still another model is where the preferred provider is employed by both donors and pharmaceutical companies when outsourcing clinical research. In this model, providers of clinical research services are selected or qualified to bid on proposals based on demonstrating their clinical research capacity. This model reduces the number of different CROs that a product developer might work with, helps ensure the quality of the research as suppliers have been pre-qualified, and can help reduce costs through agreed-upon pricing structures.
There are many examples of this preferred provider relationship being used by product developers and pharmaceutical companies. Government funding agencies now use this mechanism through what is called the Indefinite Delivery/Indefinite Quantity (IDIQ) contract. The IDIQ mechanism provides for an indefinite quantity of services during a defined period of time offered to a group of selected providers. A recent example from September 2010 is an IDIQ from the newly created Center for Global Health of the Centers for Disease Control and Prevention (CDC). This IDIQ is intended to provide technical assistance services to governments and local organizations across Africa, Asia, Latin America, Europe and the Middle East to bring additional scientific and technical assistance to the US Global Health Initiative. FHI is one of the three prime contractors to this IDIQ.
Collaborations needed for clinical research should extend beyond sponsors, CROs and sites, to include the community and patient population being studied.
How clinical trials are perceived internationally and in communities where research occurs can directly affect support for research, with fears or mis-information derailing trials just as easily as operational or scientific setbacks. (see the 2010 FHI publication Communications Handbook for Clinical Trials)
In 2004, for example, controversy over a planned clinical trial to test oral tenofovir in Cambodia as a potential once-a-day pill to prevent HIV forced the early abandonment of this important prevention trial. Less than a year later, similar controversy, fueled by rumours, misleading media coverage, and communications breakdowns, lead to the demise of a second HIV prevention trial in Cameroon. Together, these trials served as a wake-up call to HIV scientists and donors to re-examine the ways they communicate with local and international communities about clinical research.
When communicating about clinical research with community collaborators there is the expectation of transparency, information sharing and engagement. The HIV field is not alone in confronting changing expectations and new challenges when it comes to communicating about research. Investigators and research staff receive extensive training in GCP and specific trial protocols but are rarely trained in community engagement and communications.
Collaborative communication strategies with communities and stakeholders can help build commitment and public trust in research, create an enabling environment for work, help identify and respond to incorrect information, and encourage the uptake and eventual application of findings. Failure to attend to this new reality can occasion just the opposite: distrust, sensational or misleading media coverage, and missed opportunities to advance the research agenda.
A scan of the pharmaceutical R&D pipeline coupled with the vision of the major donors, indicates that the emphasis is shifting to preventing and eradicating disease. There are a number of promising vaccine candidates being developed for malaria, dengue fever, influenza and other infectious diseases. There is also still the hope that one day there will be a vaccine for HIV.
Disease prevention trials are extensive under takings requiring substantial investment and time. It is always desirable to conduct research studies based on best practices and previous experience. However, in the field of disease prevention trials there is an even greater need to build sound experience into the study design and implementation so as to ensure success and maximize efficient use of all resources.
Prevention science is not new and over the decades there have been many research studies aimed at understanding how to prevent disease using behavioral interventions. For example, research to assess the impact of counseling in the use of condoms and other risk-reducing measures to prevent the acquisition of sexually transmitted infections including HIV.
There are many similarities between traditional prevention and vaccine trials including:
Identification of suitable sites in both focus on incidence data and clinical infrastructure
Both often utilize healthy normal subjects who are nevertheless at high risk of the outcome
As clinical research expands, the need for collaboration by research funders and their partners, researchers, product developers, CROs and the communities becomes even more important. Collaborative and informed clinical research of the highest quality can help ensure that studies report accurate, verifiable and relevant findings making best use of available resources. Various successful models for collaboration already exist and should be considered for future research.