From mobile medical apps and fitness trackers, to software that supports clinical decisions doctors make every day, digital technology has been driving a revolution in healthcare. As we are learning to live the virtual new normal in COVID-19 era, adoption of digital solutions has witnessed further acceleration. To keep pace with this promising innovation, the regulators must modernize its approach to regulation. Question to ask ourselves is “How to ensure high-quality, safe and effective digital health products”. This article highlights changing Regulatory landscape (European/FDA Guidelines) for enabling Safe, effective, high-Quality & trustworthy AI/Digital solutions in Pharma.
From mobile medical apps and fitness trackers, to software that supports the clinical decisions that doctors make every day, digital technology has been driving a revolution in healthcare. As we learn to live in the new normal, forced by the COVID-19 pandemic, adoption of digital solutions has witnessed further acceleration.
To keep pace with this promising innovation, regulators must modernise their approach to regulation.
The question is “How can we ensure high-quality, safe, and effective digital health products”.
This article focuses on the changing Regulatory landscape (European and FDA Guidelines) for enabling safe, effective, high-quality and trustworthy Artificial Intelligence (AI) and Digital solutions in Pharma.
In the past few years, many innovations and novel approaches across Clinical Trial processes have been witnessed.
To a degree, COVID-19 has acted as a catalyst to some of these great innovations and adaptations.
Device that works with a patient’s existing device.
What we need to know is how this technological transformation impacts:
As a core part of clinical trial teams, each of us will have a significant role to play as we experience this exciting time of transformation.
We need to have a new mindset and adapt our SOPs, processes, and approach accordingly in order to accommodate these changes to the way that Clinical Trials are conducted, Data is captured, analysed, and reported.
As we adapt, we need to continue to safeguard Data Integrity, Quality, and Security by ensuring compliance to changing requirements from Global regulatory bodies.
The 21st Century Cures Act (Cures Act), signed into law on December 13, 2016, is designed to help accelerate medical product development and bring new innovations and advances to patients who need them faster and more efficiently.
As of today, more than 30 AI algorithms have been approved by the US FDA including those for the detection of diabetic retinopathy, stroke, brain haemorrhage, and atrial fibrillation.
The FDA released its Digital Health Innovation Action Plan in 2017 clarifying the FDA’s role in advancing safe and effective digital health technologies and addressing Key provisions of 21st Century Cures Act.
The FDA released its Policy for Device Software Functions and Mobile Applicationsi in 2019. This guidance explains how the Agency plans to regulate software that aids Clinical decision support (CDS), including software that utilises machine-learning-based algorithms.
The FDA is to focus regulatory oversight on higher-risk software functions, such as those used for more serious or critical health circumstances as well as Software that utilises machine-learning based algorithms, where the user might not readily understand the program’s ‘logic and inputs’ without further explanation
The Software Pre-Cert Pilot Program will help inform the development of a future regulatory model. This model will provide more streamlined and efficient regulatory oversight of software-based medical devices developed by manufacturers who have demonstrated a robust culture of quality and organisational excellence, and who are committed to monitoring real-world performance of their products once they reach the market.
The goal of the program is to have tailored, pragmatic, and least burdensome regulatory oversight that assesses organisations (both large and small) to establish trust that they have a culture of quality and organisational excellence such that they can develop high-quality Software as a Medical Device (SaMD) products Figure 1.
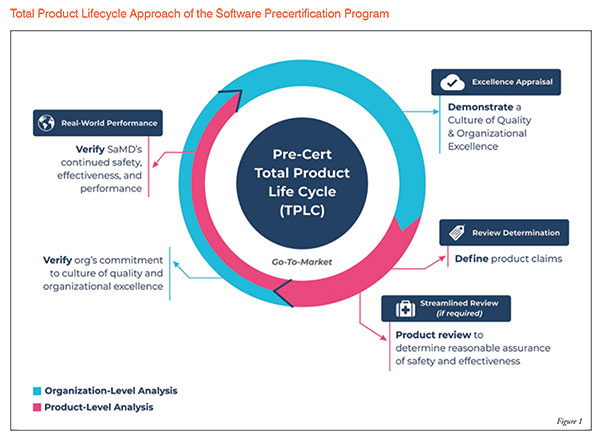
The FDA's Total Product Life Cycle (TPLC) approach enables the evaluation and monitoring of a software product from its pre-market development to post market performance, along with the continued demonstration of the organisation's excellence:
FDA Approval Status could be put at risk after each update or new iteration for AI-powered therapeutic/diagnostic tools. For example: Security Updates, adding new features or functionalities, or updating an algorithm, etc. Planning to take a version-based approach to the FDA approval process might be good idea. In this approach, a new version of software is created each time the software's internal ML algorithm(s) are trained by new set of data, with each new version being subjected to independent FDA approval.
The highly iterative, autonomous, and adaptive nature of these tools requires a new, TPLC regulatory approach that facilitates a rapid cycle of product improvement and allows these devices to continually improve, while prov Figure 2.
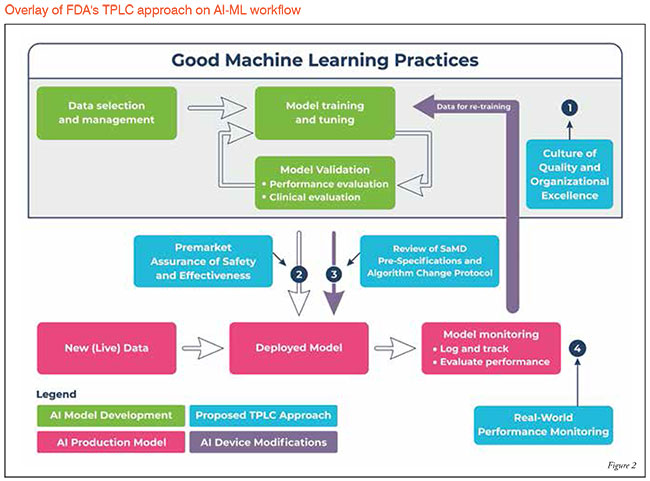
To fully realise the power of AI/ML learning algorithms, while enabling continuous improvement of their performance and limiting degradations, the FDA’s proposed TPLC approach is based on the following general principles
that balance the benefits and risks, and provide access to safe and effective AI/ML-based SaMD:
1. Setting out requirements for achieving trustworthy AI
2. European AI Alliance
3. Building international consensus for human-centric AI
IDx-DR is an AI diagnostic system that autonomously analyses images of the retina for signs of diabetic retinopathy.
This is the first ever autonomous AI system cleared by FDA (announced on 11 April 2018) that provides screening/ diagnostic decision without the need for a clinician to interpret the image or results.
This device is a software program that uses an AI Algorithm to analyse images of the eye taken with a retinal camera called a Topcon NW400. Once the Doctor uploads the digital images of the patient’s retina to a cloud server on which IDx-DR software is installed, the software provides doctors with one of two results:
With ongoing Global health concerns and the current COVID-19 pandemic situation, we need to identify and encourage forward-looking ideas, facilitate innovations, and bring them to life in order to cultivate ideas into potential solutions for our customers and, most importantly, save the lives of patients in need.
We must ensure both Patient Safety and Data Integrity when dealing with Technology.
Working professionals need to understand the changing landscape, Data sources, and Metadata, to ensure Data Quality during its processing and be able to upskill themselves to manage new technologies and tasks.
Global Regulatory bodies are actively encouraging the implementation of technology for faster drug development and are introducing Key Guidance documents and Initiatives.
Sponsors and vendors need to think innovatively in terms of their Study designs and executions plan and incorporate technology driven elements. Only then can we develop innovations beyond the current boundaries.