Formulators are faced with an increasingly competitive industrial environment and very challenging compound properties. Smart development strategies, like frontloading are needed in order to increase the R&D productivity and to bring new chemical entities earlier onto the market. Key factor for success is the intensified cooperation between research and development.
The duration of drug development cycles is continuously being challenged by an increasingly competitive industrial environment. The number of drug candidates intended for pre-clinical and clinical development has dramatically increased by using modern high-throughput technologies, such as combinatorial chemistry and pharmacological screening. However, in most of the new compound selection processes an increasing number of new chemical entities were found to be showing poor aqueous solubility and poor membrane permeability. This is due to the fact that the compounds are getting more and more difficult to formulate and at the same time, the time available to develop these drug candidates is continuously reduced.
This presents a major contradiction: In a consistently decreasing process time, an increasing number of complex drug candidates have to be formulated in order to support pre-clinical programmes as well as human clinical studies.
In order to overcome this contradiction, smart development strategies are needed to maintain or even shorten the overall development timelines and to address the compound related issues adequately and in a timely manner. Moreover, in early development, aspects such as upscalability and cost of goods, which are of primary importance in commercial manufacturing, need to be considered. The common goal of the pharmaceutical industry is to bring new chemical entities earlier onto the market and to be able to reap the benefits of the patent protection of a new drug.
At Solvay Pharmaceuticals, this need is approached through an integrated analytical, chemical and pharmaceutical development. All disciplines contribute to the success equally. An insight into the implementation of the frontloading strategy with a special focus on the implementation of new formulation development strategy is discussed here.
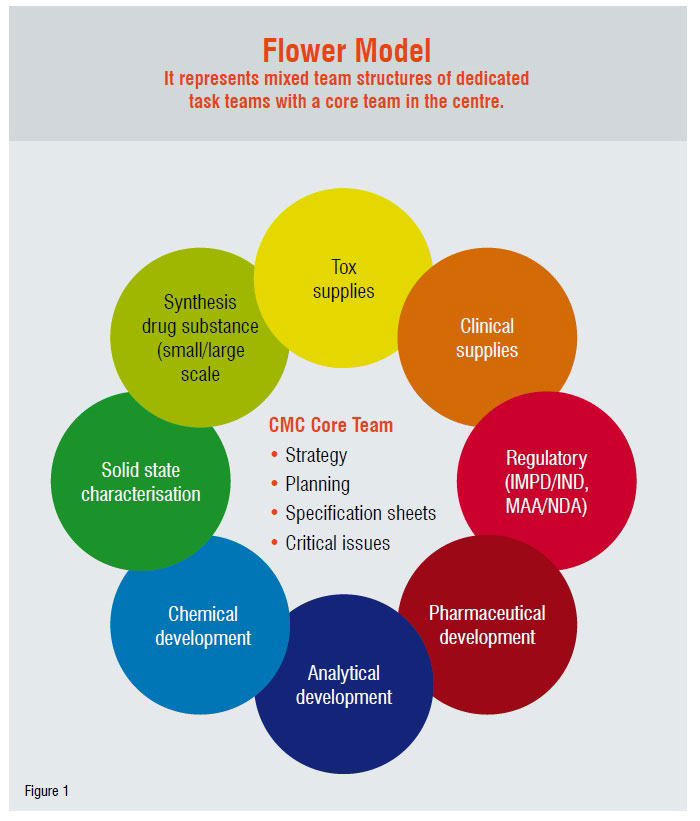
One of the most important requirements to increase the R&D effectiveness is an improvement of the intra- andinterdisciplinary communication. A strategy change can only be successfully implemented when the walls between the “silos” (i.e. the different disciplines) are successfully broken down. At Solvay Pharmaceuticals, this is achieved by having mixed team structures. A core team of scientists from various disciplines is responsible for the chemical and pharmaceutical development activities of a compound. The tasks are performed by interdisciplinary task teams. That enables a very good knowledge sharing among and inputs from all disciplines. Representatives of the task teams form the core team and it is mainly responsible for the strategy, planning and interaction between the task teams (Figure 1).
The implementation of a biopharmaceutical platform has improved the communication between different departments and disciplines even further. The platform helps to bring the right people together and to coordinate the data sharing among Research, Chemical & Pharmaceutical Development and Clinical Pharmacology departments. The data sharing aims at making available of all the data related to the compound to the team members so that the efficient evaluation of the compound characteristics can be done based on all relevant in vitro and in vivo data. This is a key requirement for enabling right decisions at the right time.
It is very important that all disciplines contribute to the success equally. A shortening of development timelines requires a smarter chemical development, as well as an improved analytical and formulation development.
In the past, a serial approach was followed from the lead candidates towards the market formulation. In general, the good water-soluble and good permeable compounds (defined as BCS class 1 compounds according to the well-known Biopharmaceutics Classification System) did not require intensive formulation efforts to become bioavailable. Therefore, the formulations for these compounds used in pre-clinical and clinical programmes were relatively simple, like suspensions of micronised API—in capsules or even just plain solutions. The real formulation development including process development was started after a proof-of-principle study in patients was successfully completed (i.e. clinical phase IIa).
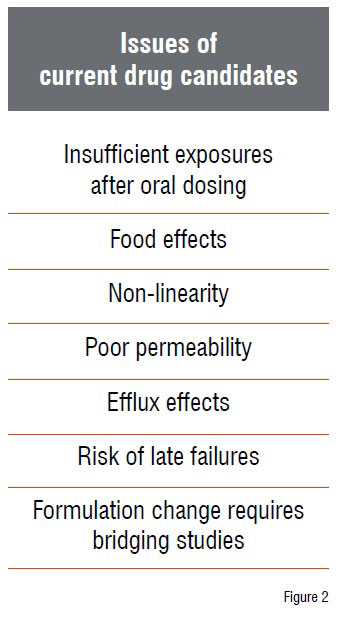
As long as the compounds belong to BCS class 1, the conventional approach is very effective and also reduces costs. However, this approach does not work very well for more challenging compounds from the BCS classes 2, 3 and 4. The administration of such compounds is connected with a lot of different issues as shown in Figure 2.
If such complex compounds are administered as standard formulations, often enormous API loadings are required to reach sufficient blood levels. Normally, the formulators are then asked to come up with enabling formulations to decrease the doses or to make the formulations more attractive for marketing. The formulation changes require bridging studies. There is a high risk of late failure when no suitable formulation approaches can be found to solve the issues. All the above-mentioned facts necessitate the implementation of a new strategy.
It is therefore clear that only by putting more efforts into the processes would one be able to minimise the risk of inadequate exposure when formulating problematic compounds. The key question to answer while formulating complex compounds is which of them has the potential to be the most promising drug candidate that can be taken further into the full development programmes. Therefore, it is necessary to develop different formulation approaches in parallel and to test the different formulations in suitable in vitro and in vivo test systems. The aim of all these efforts is the identification of the optimal formulation principle for the specific API as early as possible. This lead formulation principle can then be maintained for this specific API throughout all pre-clinical and clinical phases. In some cases it will be even possible to identify two almost equally suitable formulation approaches. In this case it would be possible to select the lead formulation approach and to keep a potential backup formulation for the life cycle management of the drug. The earlier the lead formulation principle can be identified, the more time will be available for the optimisation of the formulation including, for instance, the Quality by Design activities and process optimisation. This is of particular interest if a relatively new formulation approach has to be applied in an industrial scale setting.
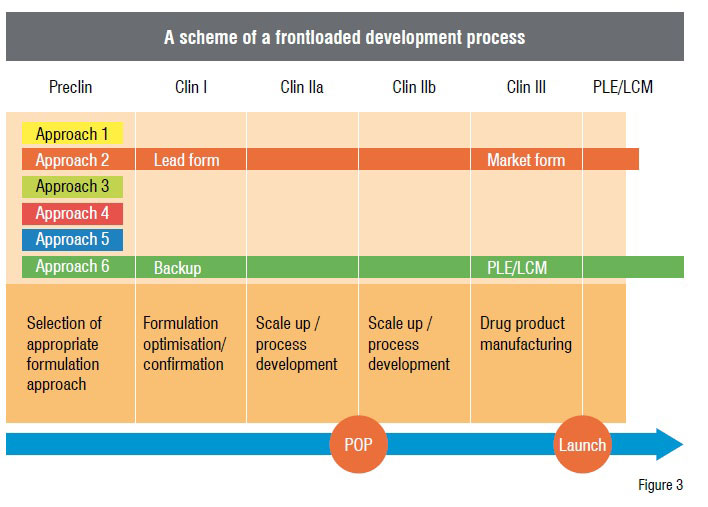
There is also another big advantage of using this strategy. If it is not possible to find a suitable formulation approach during the early stages, then there is very little chance that this formulation approach can be found later. Hence, the development of such compounds could be discontinued as early as possible. Resources thus saved could be used for more promising drug candidates. Furthermore, the medicinal chemists could try to improve the compound characteristics, if the underlying reasons for the low bioavailability are elucidated. When comparing both the strategies, it becomes obvious that in the new approach many development activities are shifted towards a very early stage of the process. Hence, this approach is termed as frontloading (Figure 3).
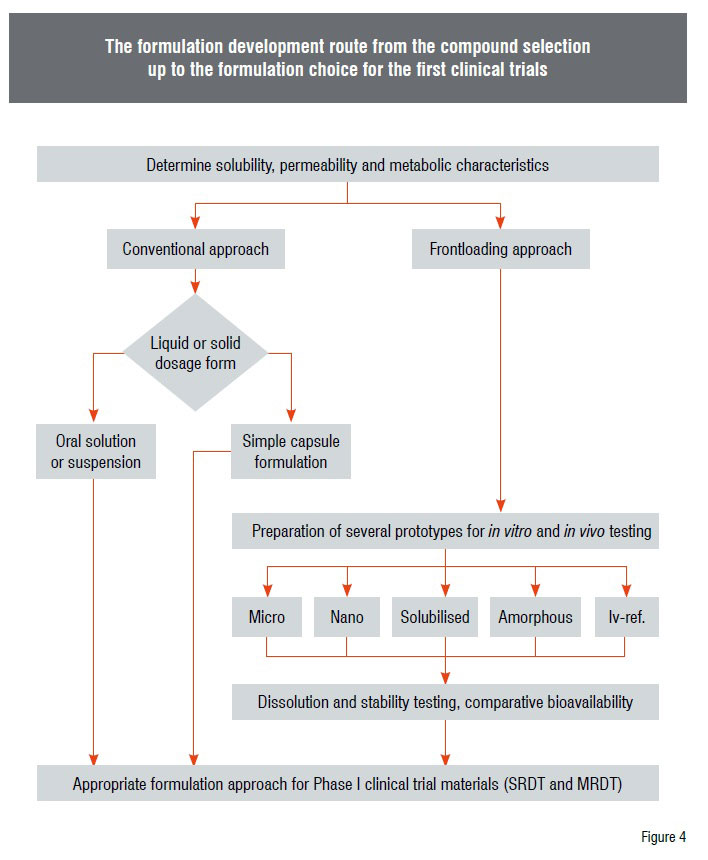
The implementation of the strategy in Solvay Pharmaceuticals started with considerations as to how the most appropriate formulation approach can be selected in an effective manner. It should not be forgotten, that the goal of this strategy is to shorten the overall development timelines. When a new compound arises it is first classified with regard to its aqueous solubility and its permeability. If the compound can be regarded as BCS class 1 compound the formulation should be as simple as possible, comparable to the conventional approaches. However, if a compound shows solubility in aqueous media of lower than 40µg per mL and/or a very low permeability or a high metabolism rate, determined through in vitro tests, then it is a candidate for the full blown formulation approach. That means that different formulation types are developed in parallel. This multi-tier approach comprises many vitro tests, like discriminating dissolution tests, short-term stability test of prototype formulations (Figure 4).
The low bioavailability of a compound can be in general either due to low solubility, dissolution or permeability rates.
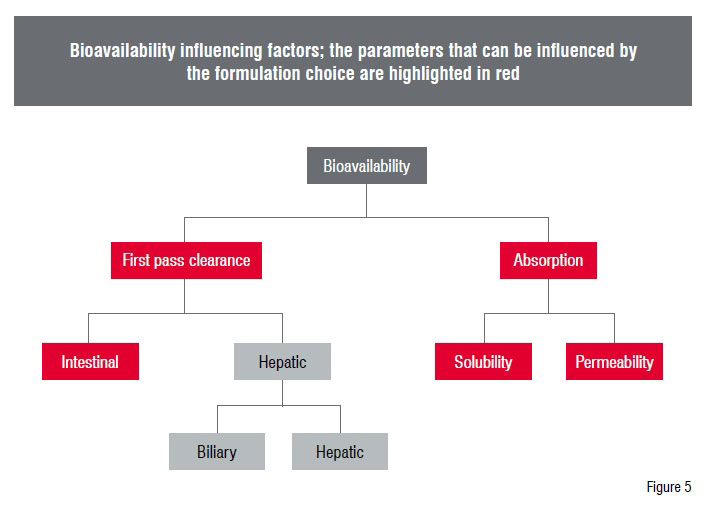
In some cases it can also be a combination of the different factors leading to a very low bioavailability. A very high first pass clearance could be another reason for low blood concentrations. In this case the tools of formulation developers to improve the bioavailability are relatively limited. Normally, the influence of the formulation ends with the intestinal absorption. A hepatic first pass effect is therefore out of the range of formulation improvement. However, solubility related issues, and to a minor extent even permeability related issues, can be addressed and improved with suitable formulations.
Particle size reduction, solid state manipulation, solubilisation and permeation enhancement are the main techniques toimprove the bioavailability of problematic drug candidates within Solvay Pharmaceuticals. A comparison of the influence of the first three approaches can elucidate solubility rate and dissolution rate limited bioavailabilities. The permeation enhancing strategies are of particular interest for BCS class III compounds, but can also be used for BCS class 4 compounds.

The approaches should be as universal as possible, meaning that one should be able to apply this formulation principle for liquid formulations as well as solid dosage forms. An example of a universal formulation approach is given in Figure 6. For instance, the formulation approach particle size reduction can be applied as nanosuspension when a flexible dosing or a special administration route is required. However, the nanosuspension can also be transferred into solid dosage forms, like capsules or tablets. The formulation principle in this case would be particle size reduction or nanonisation.
This part of the development ends normally with a comparative PK study of different optimised prototype formulations in a suitable species. Normally, this comparative bioavailability study enables the formulators to select the most promising formulation approach with regard to predefined parameters like developability, patentability, scalability, cost of goods, stability and bioavailability improvement.
The limited availability of API of sufficient quality is one major obstacle for the development of different formulations in parallel. It is clear that formulation development especially during the early drug discovery and lead optimisation phase is always a balance between rapid screening and commercially viable formulation development. Frontloading certainly requires more API at earlier stages. This needs to be addressed by the chemists.
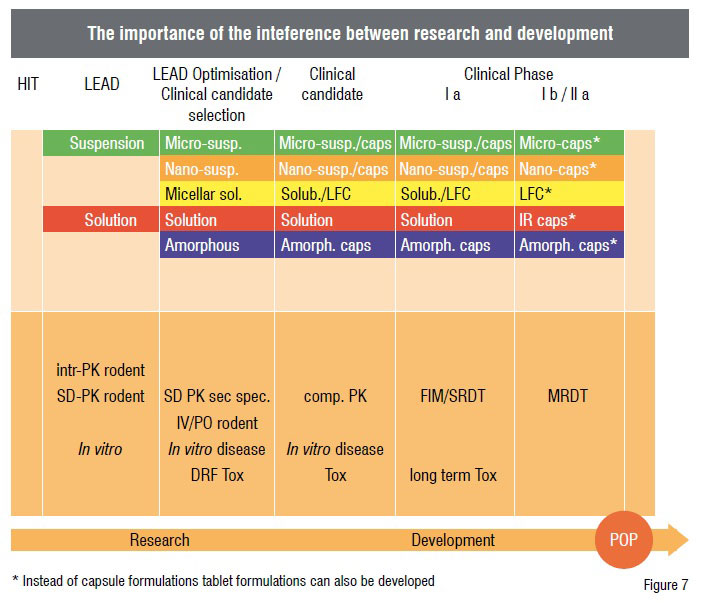
However, the formulation scientists are also asked to improve their experiments in such a way that less API is needed in order to save costs. Therefore, the miniaturisation of the experiments is an important requirement to start more activities earlier. Furthermore, it is important to avoid an unnecessary replication of experiments. All formulation activities from the lead candidate phase until the clinical phases have to be linked with one another. Figure 7 shows the ideal situation for a poorly soluble compound in which the formulation selection and optimisation process starts during the lead phase, when normally only very tiny amounts of API are available. For that reason, only two different approaches are tested in vivo at this stage. The comparison of the bioavailability obtained from, e.g. a suspension or a solubilised system, can give important guidance for the subsequent development steps. During the lead optimisation phase when more API is available, the number of different formulation approaches to be tested can be increased. The figure also shows that it is possible to transform liquid formulations into solid dosage forms while maintaining the formulation principle. Once the lead formulation approach and a potential backup formulation approach are chosen, it is no longer necessary to develop the unsuccessful approaches further. The parallel approach is also beneficial for the biological testing.
Suitable formulations can be chosen from the portfolio with respect to the kind of the in vivo study, for instance the requirements for long-term toxicity studies are different from that of explorative PK studies. However, it is necessary that formulations used within the enabling toxicity study reflect the properties of the lead formulation approach considered for the clinical trial material.
The strategy change described in this article requires a change in the mindsets of the people involved in the drug development process. Every member of the organisation has to be involved in the process. Frontloading can lead to an accelerated development process, if smarter techniques are applied consistently from all disciplines. Furthermore it is important to miniaturise the experiments in order to reduce the required drug amounts. An integrated development approach including the right in vivo studies can enable the formulator to decide on suitable formulation approaches very early on. The frontloading strategy facilitates that within shorter time periods. Even very challenging compounds can be formulated adequately to support pre-clinical programmes as well as human clinical studies. Compounds with issues that cannot be solved can be identified and discarded early in the beginning. The capacities can then be focussed on the most promising compounds. All these activities can enable the pharmaceutical industry to reach the overall goal—to launch new drugs onto the market earlier.
References
1) S. Balbach, C. Korn / International Journal of Pharmaceutics 275 (2004) 1–12
2) J. Maas et al. / European Journal of Pharmaceutics and Biopharmaceutics 66 (2007) 1–10
3) Dhiren R. Thakker, Strategic use of preclinical pharmacokinetic studies and in vitro models in optimizing ADME properties of lead compounds. in Optimizing the “Drug – Like” Properties of Leads in Drug Discovery, AAPS Press, Springer 2006)