This article will describe a translational pharmacology roadmap for complex biologics in early development, spanning new molecular entity declaration to Phase 1 clinical study design, and including milestone activities such as developing a target product profile, evaluating first-in-human risk, and selecting the first-in-human starting dose.
What is a Complex Biologic? Biologics are drugs isolated from natural sources, and include proteins, nucleic acids, viruses and cells. Biologics are usually complex mixtures produced by biotechnology which are not as easily characterised or manufactured as small molecule drugs. They are typically administered parenterally or subcutaneously to bypass degradation in the gastrointestinal tract. Complex biologics have development complexity arising from novelty of the platform, target(s), mechanism(s), nonclinical to clinical translation, manufacturing, or development strategy. Examples include bi-specific platforms, Chimeric Antigen Receptor (CAR) T-cell therapies, vaccines, Antibody-Drug Conjugates (ADCs), and gene therapies.
A few important milestones that need to be met during drug development are New Molecular Entity (NME) declaration, pre-Investigational New Drug (IND) or Preclinical Trial Application (CTA) meeting, and IND/CTA submission. In preparation for these milestones, it is critically important to prospectively establish a preclinical / translational strategy to answer critical questions related to biology, pharmacology, and toxicology, which will drive formation of a strong clinical development plan. What data are required to move your therapeutics through these milestones? How are you going to collect these data? When do you need them? For complex biologics, answering common preclinical development questions often involves determining what to do in the absence of certain data or models. Much like painting a picture, the integrated body of data will point to the answer(s).
The Target Product Profile (TPP) is a dynamic resource that serves to guide development decisions across functional areas by creating alignment around attributes and outcomes for a product candidate. The TPP should be considered a living document that evolves throughout development as additional information is generated and integrated.
The goal of an FIH risk assessment is to understand potential safety concerns relating to administration of the therapeutic and help guide the starting dose selection strategy. To determine the level of risk associated with a compound, drug and system characteristics should be considered, including novelty, mechanism, potency, specificity, species or model relevance, safety findings, pharmacodynamics (PD), time course and reversibility of effects, off-target effects, exposure-response and exposure-safety relationships, and translatability. Critical questions including, but not limited to, the following list should be answered to guide the risk assessment for your candidate product:
By considering these characteristics, you can develop a sense of the overall relative risk (low, moderate or high) for a drug being administered to humans for the first time and can then implement strategies to mitigate specific risks in both clinical dose selection and study design. A deep understanding of the molecular engineering, biology, toxicology, pharmacology and translational science is required to properly evaluate risk in an FIH study, and this requires cross-functional team integration across areas of expertise.
Selecting the FIH starting dose is a truly difficult aspect of preclinical development. Complex biologics, especially those that modulate the immune system, have the potential for exaggerated and unexpected pharmacology. Preclinical models often fail to predict the potential for human toxicity. They may also underestimate or fail to predict beneficial immune modulation.
The major dose selection methods include:
Typically, more than one method is used to calculate potential starting doses and knowledge of similar drugs is also considered; integration across approaches results in triangulation on the selected (optimal) starting dose. A starting dose calculated from MABEL approaches will usually be lower than a predicted optimal biologic dose. The lowest starting dose, while safe, is not always the best choice.
In the case of a low-risk complex biologic, such as for a well-understood target, platform, or mechanism with substantial clinical precedent, a higher starting dose using a toxicology-based approach may be acceptable. Use of NOAEL or HNSTD requires appropriate toxicology species and justification. Following conversion of the NOAEL dose into an HED, a safety factor of 10 or other multiplier may be added to account for differences in affinity, expression, or other unknowns.
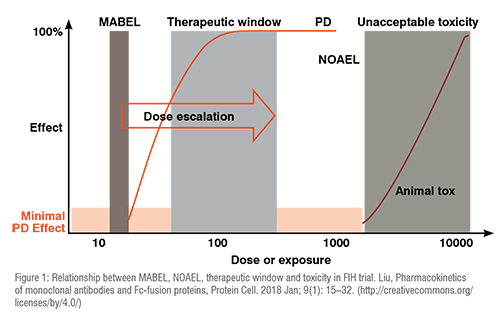
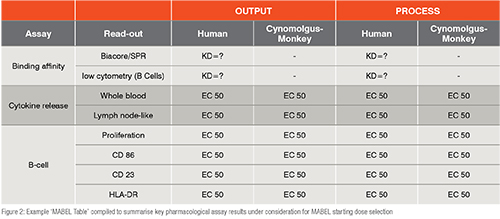
In the case of high-risk complex biologics, an in-vitro calculated MABEL is likely most relevant for dose selection. Certain drug candidates may be considered high risk for multiple reasons, including a possibility of unexpected cytokine release, a novel mechanism of action, or difficulty in calculating an FIH dose due to limited data. A master ‘MABEL Table’ is recommended to summarise the key pharmacological assay results under consideration for MABEL or modified MABEL starting dose selection (see Figure 2). Notably, this table can enable meaningful comparisons across assays as well as with comparator molecules. For MABEL calculation, relevant assays should be considered, which include those that are closest to the mechanism of action. The selected assay is typically the most sensitive of the parameters relevant to the mechanism of action. Once the FIH MABEL dose is calculated, the team must then also consider the feasibility of formulating and administering the MABEL starting dose to patients as well as whether the MABEL dose will provide any clinical benefit.
In the case of moderate- to high-risk biologics, a modified MABEL approach may be employed, which often uses in-vivo RO to understand the proportion of a pharmacological target receptor that is bound by drug. In theory, the response to a drug increases with increasing RO. By establishing a human PK projection from your most relevant species, such as non-human primates, you can then link PK to the RO established in preclinical models to project human RO. The goal is to determine what human PK and what dose of drug can result in your target percent RO.
The primary objective for a Phase 1 FIH study is to demonstrate safety, and selection of a safe starting dose is critical to achieving this objective. However, clinical pharmacology strategy for biologics in early development extends beyond starting dose, particularly in oncology and other indications where Phase 1 studies are conducted in patient populations and the potential benefit must also be considered with the risk.
In addition to starting dose, other Phase 1 dosing considerations for complex biologics may include evaluating the need for repeated dosing, exploring feasible dosing intervals based on the ability to achieve PK and/or PD targets, the decision to use weight-based or flat dosing, dose escalation increments and targeted dose range based on the anticipated therapeutic window, and setting a maximum dose in the absence of dose limiting toxicity (DLT) or safety signals. As with FIH starting dose selection, the strength of these dosing recommendations will depend upon the quality of preclinical data generated to inform them, such as dose-ranging PK/PD and efficacy studies in relevant animal models.
A robust clinical pharmacology strategy also includes study design and analysis considerations such as DLT period duration, 3+3 vs adaptive design, sentinel dosing, expansion and PK/PD cohorts, strategic sampling for PK, RO, anti-drug antibody (ADA)/immunogenicity, and PD to drive robust mechanistic modelling and exploratory analyses, timing of bioanalytical assay readiness, interim cohort-by-cohort analyses, iterative population PK analyses, and early dose-safety or dose-response evaluations.
For Phase 1 combination studies, dosing considerations include combination dose and interval for all agents driven by an understanding of potential synergy and drug-drug interactions, patient-level escalation and de-escalation plans to ensure interpretability of resulting data, sequencing, duration of dosing, and intelligent sampling plans for identification of changes in PK, RO, ADA, and PD as compared with mono-therapies.
Progressing clinical development requires making important decisions often in the absence of guidance. PD biomarkers can inform those decisions if critical questions have been clearly established earlier in preclinical development. In the early development of complex biologics, where PD biomarkers are commonly included as an exploratory endpoint of Phase 1 studies, strategies which are not recommended include creating a list of biomarkers without clear rationale, implementing a nonspecific shotgun approach to investigate panels of biomarkers for a possible lead, or exclusion of biomarkers with sole reliance on outcome measures. Instead, it is crucial to leverage the expertise of translational research and pharmacology experts to design and implement intentional biology-driven biomarker strategies which can be used to enable key development decisions.
In Phase 2, the primary objective is proof of concept for efficacy, and selection of the optimal biologic dose as the Recommended Phase 2 Dose (RP2D) is critical to achieving this objective. Recommended Phase 2 dose selection considers the totality of available data, including safety, PK, PD, efficacy, modelling and simulation, and target product profile. For complex biologics, PD biomarkers are especially critical for elucidating biology to drive RP2D selection in the absence of maximum tolerated dose. Ultimately, we evaluate all the available information to try to understand drug behaviour and patient responses. The quantitative relationship between all parameters and the time course of all endpoints are used to drive rational decisions for RP2D regimen (dose and interval). As with Phase 1 dose selection, RP2D selection is like painting a picture, where the integrated body of data will point to the answer(s).
Summary
Biologics are growing in complexity. Key milestones that must be carefully considered in early development include the TPP, FIH safety risk evaluation, FIH starting dose selection, and Phase 1 study design. By using a well-designed clinical pharmacology roadmap, we can drive complex biologics toward successful marketing authorisation and bring new safer, more effective drugs to market.