Layer-by-layer encapsulation gives a very stable fine dispersion, so fine that a dollar coin is visible when looked at through the clear dispersion.
Since layer-by-layer (LbL) polyelectrolyte assembly was introduced in the 1990s by G. Decher, Y. Lvov, H. Möhwald and M. Rubner it has found application in various fields that study and apply nanotechnology. LbL nanoassembly is based on the sequential adsorption of positively and negatively charged polymers on a surface to form a nano-thick film of coating. The process is illustrated in Figure 1.
During LbL assembly oppositely charged polymers are electrostatically bound to one another producing insoluble nano-thick multilayers. The thickness of this multilayered film is controlled by the number of 2-3 nm polyelectrolyte bilayers that are deposited. Therefore, by simply alternating the adsorption of polycations and polyanions, thin organised films with a thickness ranging from of 5-500 nm that are precise to within a few nanometers, can be formed. In addition the composition of the film can be controlled and varied because a vast array of polyelectrolytes (synthetic or natural, such as polysaccharides and proteins) and even charged nanoparticles will form LbL coatings and shells. The only requirement is that the positive and negative components are alternated. This means that this relatively simple nano-scale coating process has evolved into a robust platform for the modification of material surfaces or the encapsulation of various substrates (Figure 2). So much so that since the 1994 more than ten thousand papers has been published describing various aspects and applications of the LbL method.
Experimentally various techniques are used to produce LbL films and coatings. The simplest of these is to either dip a solid object into aqueous solutions containing either polycations or polyanions or alternatively to spray the polyelectrolyte solutions onto the surface of the material to be coated. Using these methods polyelectrolyte multilayers can be formed on larger solid substrates and on very tiny colloidal particles. Colloidal coating was first reported by J. Kirkland, G. Sukhorukov, F. Caruso and H. Möhwald. They showed that a 5-50 nm thick polyelectrolyte shell can be formed on colloidal cores such as drug micro and nano particles. When coating drug particles typically a colloidal suspension of the drug is formed in a polycation solution, and after a few minutes, non-reacted polyelectrolyte is removed by centrifugation or filtration. This process is then repeated using now positively charged coated drug particles suspended in a polyanion solution. Similarly assembly is repeated until the required number of bilayers is formed around the drug core particles.
When coating drug particles a few things should be kept in mind. First, it is important to avoid drug dissolution during the coating process. This is done by adjusting the pH or to do the coating in a saturated drug solution. Secondly, although the first publications on LbL assembly used synthetic polyelectrolytes; recently most researchers interested in drug delivery use natural biodegradable compounds, such as anionic alginic acid, hyaluronic acid, chondroitin sulfate, heparin, dextran sulfate, carboxymethyl cellulose, polyglutamic acid, albumin, glucose oxidase, DNA, and cationic chitosan, dextran amine, polylysine, collagens, protamine sulfate and gelatin A. Third, once the coating process is establish for a specific drug the formulator usually pick polycation / polyanion combinations that allow control over drug release from hours and even days. However, for some drugs longer release rates requires thick 6-12 bilayer shells which could be time and labor intensive. To date the release and stability properties of the following drugs have been changed by LbL encapsulation: ibuprofen, furosemide, nifedipine, naproxen, biotin, vitamin K3, insulin, dexamethasone, tamoxifen, paclitaxel, camptothecin, and curcumin. Earlier this year the first report of changing the functional properties of a drug excipient, microcrystalline cellulose, using LbL encapsulation also appeared. However, the most exciting application of LbL nanocoating is the encapsulation of 60-200 nm diameter nanoparticles of poorly water soluble drugs. It is exciting because the thin LbL-coating provides a strong electrical surface charge (zeta-potential of ca -40 mV) for the production of stable nanocolloids while maintaining most of the increased solubility provided by the nanosized drug particles. Drug loading is also high, 50-80 wt %, and due to their small size these LbL coated drug nanocapsules can be formulated into injectable colloids and suspensions (Figure 2c). A specific application of this general technique will be discussed later.

A second less, attractive for pharmaceutics method used for the LbL encapsulation involves the formation of LbL microcapsules on sacrificed cores with diameters of 2-5 µm. The cores are then dissolved to produce empty capsules with pH sensitive shells. Water soluble drugs and proteins are then loaded into these shells through pH depended opening of pores in the capsule walls. A major drawback of this method is that drug loading is only 1-5 wt %. Notwithstanding low loading these microcapsules have been proposed for non-injectable drug delivery such as nasal delivery of LbL coated insulin. Numerous authors also claim that because of the very flexible and thin shells, such microcapsules can squeeze though the leaky capillary membranes around tumors and therefore might be used to prepare intravenous injections of anticancer and other drugs with targeted delivery to tumors (Figure 2b).
Whichever of the methods described above is used to produce LbL coated nano and micro drug particles or capsules, it faces a number of challenges:
Let us review some recent studies reporting the application of LbL coating to drug nano-formulation with an excellent chance of moving beyond clinical trials. But before looking at LbL applications, it is important to note that nano particulate drugs formulations that to date have reached the market most apply traditional manufacturing processes that have been modernied. The first group of these processes include high-pressure homogenisation, ultra-sonication and pearl/ball milling. All produce concentrated submicron particle dispersions by mechanical disintegration of insoluble drug particles. A second approach is based on various precipitation methods. Here the formation of nanoparticlesis initiated by mixing a poorly soluble drug solution in an organic solvent, such as acetone, tetrahydrofuran, DMSO or ethanol, with a water-based stabiliser. A drawback of both these approaches is the difficulty of removing or exchanging the milling or precipitation media, usually containing up to 5-10 wt % of surfactants, without negatively affecting the stability of the dispersion. Instability can cause aggregation, crystal form changes and particle growth. This is why, despite several drug nanocrystal formulations, such as Triglige, Rapamune, Emend, Tricore, Megas ES, have been approved by the FDA for medical usage, only few are commonly used in medical practice. Of these the most popular are the intramuscular injection of paliperidonepalmitate (Invega) and intravenous injection of paclitaxel (Abraxane).
Recent studies have shown that LbL coating can potentially eliminate the instabilities associated with many drug nanoparticle dispersions because in contrast to untangled surfactant stabilizers, LbL polyelectrolyte shells do not detach easily from the surface and retain integrity upon dilution in another media. This shell can therefore be designed to improve the stability of drug nanoparticles in suspension. The inner layers minimize the surface free energy, thereby preventing crystal form changes and nanoparticles coalescence, whilst the outermost layers due to their high hydrophilicity and strong surface charge enhance colloidal stability. Intermediate layers in the shells that tie the multilayer together can be designed to serve as a dissolution barrier thereby allowing modifying and controlling the release of the drug from the nanoparticles.
For example, to avoid drug nanocrystal aggregation and high loss of materials associated with centrifugation or filtration used in classical LbL techniques, we have elaborated a non-washing LbL procedure based on monitoring the particles electrical surface zeta-potential. In this process polyelectrolytes were added step-wise in small aliquots until nanoparticles’ surface charge was reversed to an opposite values (Figure 3). Neither LbL shell integrity nor thickness was compromised, but less polyelectrolyte were used in the procedure avoiding multiple centrifugations. This will prove to be very important for scaling up when, for example, expensive PEG-modified polyelectrolytes are used in pharmaceutical applications. In addition, we have also performed LbL encapsulation of drug nanoparticles under permanent ultrasonication. This further helps to keep the nanoparticles well dispersed even when their surface charge in the preparation process is low.
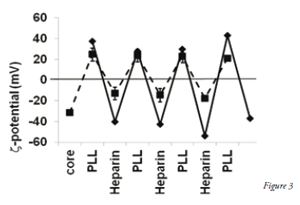
Figure 3. x-potential of two types of 170 nm paclitaxel nanoparticles coated with poly-L-lysine and heparin using the wash free LbL method. Solid line - cores prepared by powder sonication in the presence of PLL followed by LbL assembly. Dashed line –cores produced by adding alcohol paclitaxel solution to aqueous sodium docusate and Polysorbate 80 followed by LbL assembly using PLL-block-PEG.
During the coating process we also found that even better colloidal stability was obtained when at least one PEGylated polyelectrolyte was used in each bilayer, rather than coating only the outside of the capsule with the PEGylated polyelectrolyte. For example, alternate deposition of linear block-copolymers of poly-L-lysine and PEG and heparin on 170 nm paclitaxel cores allowed for the formation of stable aqueous 5 mg/mL drug colloids (Figure 4). After a three PEGylated polyelectrolyte bilayer shell was formed, the coated nano-crystals were separated from the supernatant by centrifugation. In this nanoformaltion paclitaxel concentration was 5 mg/mL which is almost two thousand times higher than its intrinsic water solubility of 0.3µg/mL. In this LbL coating process PEGylated polyelectrolyte was used because commonly used excipients such as low-molecular weight PEG and PVP do not remain within the LbL shell.
Figure 4 Right: an aqueous colloid of 5 mg/mL paclitaxel nanoparticles coated with PEGylated LbL shell, and, right: opaque paclitaxel dispersion stabilised only with surfactant. LbL encapsulation gives a very stable fine dispersion, so fine that a dollar coin is visible when looked at through the clear dispersion.
Although this new coating process has increased the applicability of LbL coating to the design of nano drug formulations an important practical question still remain. How to combine nano-sized drug dispersing processes with LbL shell assembly to produce injectable particles that are be less than 200 nm in diameter? An intriguing aspect under investigation by us is the possibility to form LbL shells on nanosized cores drug-mill where the milling medium contains a sufficient excess of uncharged pharmaceutical excipients (e.g. sucrose, glycerol, non-ionic surfactants, polysorbates, polyvinylpyrrolidone (PVP), polyethylene glycol (PEG)). The excipients, combined with ionic surfactants, are often added to increase the efficiency of the milling process and the functionality of nanosized injectable drug dispersions. Similar to the LbL process these charged amphiphiles (sodium lauryl sulfate, sodium docusate) or polymeric (carboxymethyl cellulose, chitosan) surfactants attach to the nanoparticle-during grinding and thus serves as anchors for the rest of the shell formed by LbL coating. This allows the possibility to combine in the shell components with different functionality; for example, to enrich the capsule outermost layers both with PEGylated compounds and albumin as described above during the size reduction process, e.g. milling or precipitation.

While the fundamentals of LbL shell buildup on nanocores generally follow the same rules as for larger microparticles, there are size-related limitations as the size of the cores decrease: A limited space for polyelectrolyte adsorption on one nanoparticle, and 2) influence of solution ionic strength on of polyelectrolyte tails protruding into the dispersion medium. To avoid bridging of neighbor particles and formation of grape-like structures, polyelectrolytes of less than 50 kDa molecular mass are preferable. Another complication is when transitioning from in vitro to in vivo, the colloidal stability behavior change as the particle size of the drug decreases. Many perspective drug nano-formulations end at the stage of in vitro stability testing in buffers and are never tested in vivo in animals or humans because of immediate aggregation after application. However, by combining the above described techniques, we prepared nanocolloids of several anticancer drugs coated with LbL shells of PEGylated polyelectrolytes that are stable in PBS and in serum nanoparticles. Preliminary in vivo experiments show that such shell architecture can be used in intravenously injectable formulations. In addition to PEGylation improving the in vitro and in vivo dispersion stability of LbL nanocapsules, it also prolonged their blood circulation.
In this paper, in addition to an introduction to LbL coating as applied to drug formulations, we highlighted a recent important breakthrough in LbL coating that allows for the formulation of 100-200 nm nanoparticles consisting of a core of poorly soluble anticancer drug and PEG-modified polyelectrolyte shells. These nanocapsules are much smaller than traditional LbL produced microcapsules. With this discovery we changed the usual strategy of LbL-encapsulation from making microcapsules with many layers in the shell walls for encasing highly-soluble materials to the use of very thin polycation / polyanion coatings on poorly soluble drug nanocores. We have shown that this technique for making very stable aqueous nanocolloids works well for poorly soluble drugs, such as paclitaxel, and for dyes, and even insoluble inorganic salts. This approach uses different PEGylated polyelectrolytes that not only provide good physical stability to the nanocolloids at much higher concentrations, but also improves the in vivo performance (for example, better than currently available formulations of the drug paclitaxel). Recently, two of us (MD and YL) edited a special issue of Advanced Drug Delivery Reviews, 2011, v.63, August issue, pp. 701-980 on LbL nanoshells were the reader can find additional information on the topic.
This work was supported by Award R01CA134951 from the US National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institute of Health.