Application of Lean Sigma to pharmaceutical research and development is a scientific process and requires high level of engagement from all key stakeholders.

The pharmaceutical industry now operates in a complex and dynamic global environment. The pace of change is much faster and far less predictable than ever before. To survive and prosper in today's business environment we need to urgently rethink about how we operate. Specifically, we must challenge the thinking that created our current state and develop new thinking to design our future. To have a secure future we must become capable of being much more responsive to the changing environment and be more efficient in our internal processes. We must become Lean and agile. Lean Sigma is a highly structured customer-focussed, and data-driven problem-solving approach that has been applied with great success to process and business improvement across many industries. Following is a brief of our new thinking developed from our recent experiences in the application of Lean Sigma to meet the challenges of pharmaceutical R&D improvement.
At AstraZeneca Pharmaceuticals, putting patients' health first is the foundation for all we do. Our scientists share a common goal: getting new life-changing medicines to patients as quickly and safely as possible. To do this our R&D processes deliver value to patients through a professional project management organisation supported by key disciplinary functions. These cross-functional project teams build and guide our new product development towards our goal.
As our scientists like to tell us "We are not Toyota!" It is true that application of Lean Sigma to the world of pharmaceutical R&D required us to think through how we adapt this approach, which in part emerged from its manufacturing roots in Toyota's Production System. To understand this, it is imperative to compare the pharmaceutical industry with the auto industry in terms of our R&D processes.
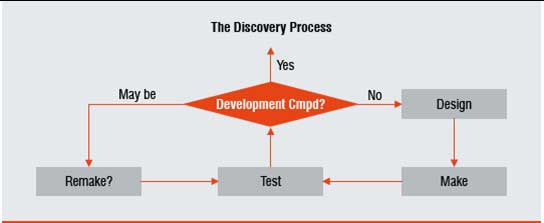
Figure 1: The core DESIGN-MAKE-TEST R&D discovery process
There are some clear differences as well as similarities between the R&D processes of the two industries. For example, the auto industry produces cars that deliver value to customers in terms of the functions they provide i.e. mobility, status, entertainment etc. The pharmaceutical industry delivers medicines to patients that provide value and improve health and ultimately the quality of their life. In the auto industry value is created by building a "machine" (i.e. a car) using engineering design concepts. In the pharmaceutical industry value is created by developing new medicines using the process of scientific discovery.
The core R&D process in both industries is similar and may be described as DESIGN-MAKE-TEST (Figure 1). In this three-step process, engineers or scientists begin their work driven by some perceived customer need to creatively craft (DESIGN) early versions or subcomponents of their product. Building prototypes (MAKE) then allows them to see if their initial ideas worked as planned (TEST). The results from the tests provide learning that is fed back into DESIGN for a second cycle of the process. The process cycles until a potentially viable new medicine or a new car emerges for full-scale development. Both industries work to meet or exceed the requirements of the relevant regulatory authorities prior to launching their products in their respective markets.
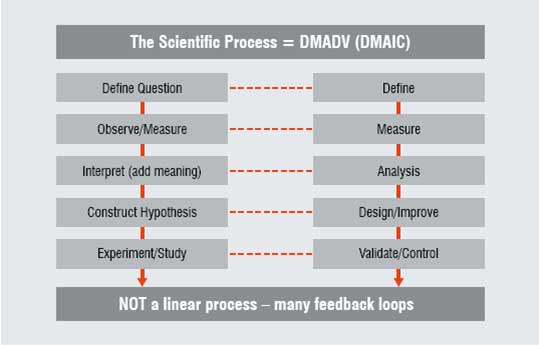
Figure 2: Comparison of the scientific process with the core process of lean sigma
The nature of a "Design" is different in the two industries. In the auto industry, it can take the form of a blueprint of a car or an engine. In the pharmaceutical industry, a "Design" may refer to the chemical structure of the bioactive agent. It is at this level we begin to see the major differences in the nature of the R&D processes of the two industries. A car is designed to work within a relatively well-understood system (described by Newton's Laws of Motion) whereas a medicine is designed to work within a poorly understood system (i.e. a disease state of the human biological system). The former applies knowledge from Physics that is relatively predictable, the latter uses knowledge from Biology and Chemistry that is much less predictable. It is this difference in the level of accessible knowledge between the two industries that necessitates a modification of how we apply Lean Sigma.
Science is concerned with the acquisition of knowledge and it follows similar processes around the world. Interestingly, there are significant parallels between this process and the core processes of Lean Sigma (DMAIC - Define, Measure, Analyse, Improve, Control) and Design for Six Sigma (DMADV - Define, Measure, Analyse, Design, Validate).
Application of Lean Sigma to R&D is arguably simply an application of the scientific process to the core R&D processes. As such, we can see that one way of "improving" the science we use is to accelerate the acquisition of knowledge in the DESIGN-MAKE-TEST cycle.
When we apply Lean Sigma to the iterative DESIGN-MAKE-TEST process, one question we face is what is actually flowing in our processes? In an early example from our work, we mapped the complex cross-functional lead optimisation process. This is the last stage of our discovery process that generates a potential drug candidate for clinical development. At first glance we see that chemical compounds are synthesised and tested to profile their physiochemical and biological properties. These compounds "flow" from chemists to biologists. Knowledge and information "flows" into our molecular designs and is also created in biological assays. In both cases, we quickly begin to see that these "flows" are often significantly interrupted in practice leading to delays. These delays lead to many issues. One significant consequence of these delays is to slow the pace of learning for our scientists.
For example, we could see from our process mapping work and process measurement activities that the compound "flows" were often interrupted because of depletion of compound supply. Chemical synthesis as commonly practiced does not allow material to be made on demand (without a significant lead time) and so small batches of material are made to support several assays before resynthesis is necessary. Making large batches will allow many assays to be done without interruption. Unfortunately, this leads to the wastage of material since most molecules synthesised do not make it past the first few assays. The key customers for these processes-our discovery project leaders-made it clear that what they wanted was sufficient material to progress to a key late-stage assay without interruption. This led to a new process target for the amount of compound made for the first time by our chemists. It was improvements such as this and other associated improvements that led to a significant (more than 50 per cent) acceleration in the timelines of our lead optimisation projects. A reduction in the need for resynthesis gave our scientists more time for creative thinking.
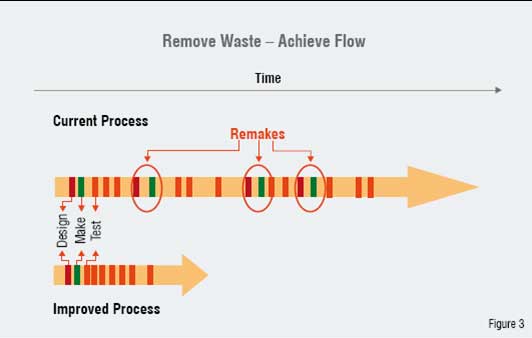
Figure 3: Schematic illustration of the approach used to eliminate waste and create flow in the iterative DESIGN-MAKE-TEST process used in lead optimisation
It is very important to appreciate that in R&D people primarily create value both individually as well as collectively in their heads in the way they think. A very important aspect of this comes from interactions between team members in our multidisciplinary project teams to develop shared meaning. Our scientists are not simply observers of the "process"-they are the process! Lean Sigma emerged from a manufacturing environment where we are often dealing with processes in which machines and / or the movement of "widgets" is a major component. The complexity of the R&D environment necessitates that we incorporate Systems Thinking alongside the more common analytical thinking mode used in Lean Sigma work. Many of our processes are far from linear and may be best thought of as being complex systems in which processes provide a means of dynamic interaction between the components.
One simple example of applying Systems Thinking to our Lean Sigma work is to recognise that cause and effect patterns are usually better described as being like a circle than a straight line pointing in one direction. A tool commonly used at the beginning of a Lean Sigma project is called SIPOC (Suppliers, Inputs, Process, Outputs, Customers), which attempts to represent the process being studied at a high level as a unidirectional flow of value towards the customer. In practice, this can only work well if there is a counterflow of information to the suppliers so that they can provide their process inputs reliably and accurately. In the lead optimisation work each component of our discovery system interacts with several other components, often bidirectionally.
The complex R&D systems required to deliver new medicines can be approached using a mental model of a "machine" in which we diagnose issues and problems as a mechanic would diagnose an engine problem. We then proceed to fix the problem as if we were external to the system. This way of thinking works well in highly controlled environments (e.g. by isolating small simple components of a larger process) but it may not be ideal to address some of the more important system-level issues. In the latter case, an alternative mental model is that of a biological (or social) system. Using this kind of thinking brings human behaviour to the fore in which people are a key part of the process-not outside of it. This type of thinking also recognises the existence of complex non-linear processes driven by numerous process feedback loops. For example, small effects in such systems can be amplified over time by reinforcing feedback loops only to reach over time a limit imposed by another type of feedback (balancing).
Approaching such systems requires a more flexible approach than simply fixing problems. It requires that we think about planting the seeds of change and nurturing change in a productive direction. In this way one should often need to think more like gardeners than mechanics! A simple example of this way of thinking is to appreciate that people (unlike machines) always have a choice and so cannot be "controlled" as a machine can. They can however be influenced and strong leadership and the understanding of human behaviour becomes an essential component of successful Lean Sigma projects.
We have found that success in our Lean Sigma work requires a high level of engagement of all key stakeholders in a process. Interestingly, scientists are often trained to be highly independent and sceptical of new approaches to improving R&D, but once they experience Lean Sigma they begin to see the parallels with the scientific process. At this point they can become powerful forces for spreading this powerful improvement approach.
Our Lean Sigma journey has just begun and we see many challenges as well as opportunities ahead. One major challenge is how to spread improvements and new thinking among our R&D employees (numbering more than 10,000 globally) in an efficient and effective way. We are faced with many highly variable and slowly recurring processes and a history of many initiatives and change programmes that have left many of our employees desensitised to new approaches. In addition, the rapidly changing external environment shows no sign of slowing down. Against these and many other challenges are some very exciting opportunities: