The ongoing merger of nanoscience with mitochondrial medicine gives rise to novel strategies for diagnosis and therapy of mitochondrial disorders.
Significant progress has been made in elucidating the central role of mitochondria in influencing the life and death of a cell. Yet, effective therapies for mitochondrial diseases remain elusive. However, the ongoing merger of nanoscience with mitochondrial medicine gives rise to novel strategies for diagnosis and therapy of mitochondrial disorders.
The mitochondrion as the "power house" of the cell is essential for energy metabolism. And as the "cell's arsenal" this organelle is crucial to the regulation and execution of programmed cell death ("apoptosis"). Further, mitochondria are critically involved in modulating the intracellular calcium concentration, which in turn impacts almost the entire cellular biochemistry. The mitochondrial respiratory chain (thought to be contributor to ageing) is the major source for damaging reactive oxygen species and mitochondria play a crucial role in numerous catabolic and anabolic cellular pathways.
The number of mitochondria per single cell generally depends on the cell's energy demand. Metabolically active organs, such as the brain, liver, cardiac and skeletal muscle tissues contain cells with a large number of mitochondria, while cells needing less energy contain only a few dozens of them.
Mitochondria form a complex tubular network that constantly changes shape by undergoing fission and fusion as shown by some recent work. Interestingly, this network has also been shown to be tightly intertwined with cytosolic microtubule. These new insights into the mitochondrial morphology have far reaching consequences in the design of future strategies for the delivery of drugs and DNA to and into mitochondria.
Mitochondria are unique in comparison to other animal cell organelles. Mitochondria contain their own genome (mtDNA) and associated machinery for the synthesis of mitochondrial proteins, all of which are crucially involved in the process of converting food into metabolic energy. Any mtDNA defect eventually reduces the energy production of this organelle. Clinical symptoms vary with the magnitude of damage and depend on the energy demand of the corresponding tissue. The causal link between mtDNA defects and human diseases was described for the first time in 1988. Since then the number of diseases identified to be caused by defective mtDNA has skyrocketed. To date, 347 distinct mitochondrial disorders have been recognised. The majority of these disorders exhibit either neurodegenerative or neuromuscular pathologies.
According to an estimate made by the United Mitochondrial Disease Foundation (UMDF), a child born every 15 minutes either suffers from a mitochondrial disease or will develop one by the age of five. Unfortunately, despite the staggering occurrence of mitochondrial diseases they are generally underdiagnosed or misdiagnosed.
They are characterised by a bewildering array of signs and symptoms. For example, one single point mutation in mitochondrial DNA has been reported to lead to over nine different disorders, including diabetes, congestive heart failure, chronic progressive external ophthalmoplegia (CPEO), schizophrenia and kidney malfunction.
Mitochondrial medicine is a rapidly growing area in biomedical research giving rise to the development of new sub-disciplines such as Mitochondrial Pharmacology and Mitochondrial Pharmaceutics. And providing the much needed tools for probing, accessing and manipulating mitochondria as well as their sub-organellar components is the ongoing incursion of nanoscience into the realm of mitochondrial research.
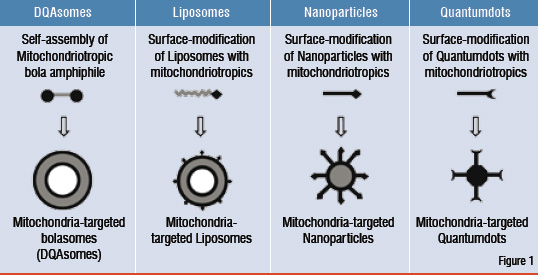
Figure1: Mitochondria-specific Nanotechnology
Figure 1 displays different types of nanovesicles and particles, generally referred to as nanotechnology, that have been designed to manipulate or probe mitochondrial functions.
The majority of these systems employ active targeting mechanisms. Their mitochondria-targeting capability is primarily achieved through association with "mitochondriotropic" residues. Further, mitochondrial leader sequence peptides and antibodies also provide for alternative targeting mechanisms.
Through intrinsic design or surface modification, these nanosystems exhibit mitochondriotropism and represent the convergence of nanotechnology with the many facets of mitochondrial research.
DQAsomes (i.e. dequalinium-basedliposome-like vesicles), proposed in 1998, represent the prototype of nano-scale mitochondria-specific drug delivery systems. The design of these unique mitochondria-targeted drug carriers is based on the intrinsic mitochondriotropism of dequalinium and its derivatives. Prerequisite for creating this system was the distinct self-assembly behaviour of dicationic quinolinium derivatives. These are mitochondriotropic cations resembling "bola"-form electrolytes, i.e. they are symmetrical molecules with two charge centres separated by a hydrophobic chain at a relatively large distance. Such "bola"-form like amphiphiles form upon sonication of aqueous suspensions cationic vesicles ("bolasomes") termed "DQAsomes" when prepared from dequalinium salts.
DQAsomes represent the first vector for selectively delivering DNA to mitochondria within living mammalian cells thereby making mitochondrial gene therapy at least feasible. DQAsomes are also being explored as mitochondria-targeted carriers for small molecules, for example drugs that are able to trigger apoptosis via interacting with mitochondria.
Such approach will eventually make it possible to "talk" cancer cells normally resistant to programmed cell death "into committing suicide". In vitro and in vivo data from ongoing investigations in the author's laboratory demonstrate that encapsulating pro-apoptotic drugs into this mitochondria-specific nano drug delivery system increases the sub-cellular (mitochondrial) bioavailability of this drug, which in turn increases the drugs' therapeutic efficiency.
For example, DQAsomal encapsulated paclitaxel triggers apoptosis at paclitaxel concentrations, at which the free drug does not have a significant cytotoxic effect. Human colon cancer cells were incubated with DQAsomal encapsulated drug and for control also with the free drug, with empty DQAsomes and with a mechanical mixture of the free drug and empty DQAsomes.
Following the staining of the treated cells with the DNA-binding fluorophore Hoechst 33258, apoptotic nuclei showing the typical apoptotic condensation and fragmentation of chromatin were counted and expressed as a percent of the total number of nuclei. Figure 2 shows that under identical incubation conditions, 10 nM paclitaxel encapsulated in DQAsomes more than doubles the number of apoptotic nuclei in comparison to the control, in which cells were treated with a mixture of empty DQAsomes and 10 nM free paclitaxel. Likewise, a DNA ladder caused by DNA fragmentation typical for apoptosis could be detected upon incubation of colon cancer cells with 10 nM DQAsomal encapsulated paclitaxel, but not upon incubation with the free drug either alone or in mixture with empty DQAsomes.
Phospholipid vesicles (liposomes) were described for the first time in 1965 by Alex Bangham. Their enormous potential as a colloidal drug and DNA delivery system for biomedical applications was discussed in two prescient papers published in 1976 in the New England Journal of Medicine by Gregory Gregoriadis. DOXILTM, liposomal encapsulated doxorubicin, was approved by the US FDA in November 1995 and represents the first injectable nanomedicine ever developed. Liposome technology has had addressed and solved issues and problems associated with the clinical application of solid nanoparticles such as biodistribution and drug release long before the term "nano" became trendy for pharmaceutical scientists.
Utilising established procedures for the surface modification of lipid vesicles, established mitochondriotropic triphenylphosphonium cations have been used to render mitochondria-specific liposomes.
Extended confocal fluorescence microscopic studies demonstrate the ability of these mitochondriotropic liposomes to deliver hydrophobic drugs almost exclusively to mitochondria. Studies with drugs known to trigger apoptosis by acting on mitochondrial targets show that delivering these drugs directly to mitochondria by encapsulating them in mitochondria-targeted liposomes significantly increases their therapeutic efficiency. For example, in vitro studies show that ceramide encapsulated in mitochondriotropic liposomes triggers apoptosis at a concentration at which the free drug does not show any toxicity. Besides the huge potential such liposomes have for the development of novel anti-cancer chemotherapies, they may also form the basis for future mitochondrial gene therapies. Most recent and still unpublished studies in the author's laboratory strongly suggest the ability of mitochondriotropic liposomes to transfer functional DNA into the mitochondrial matrix.
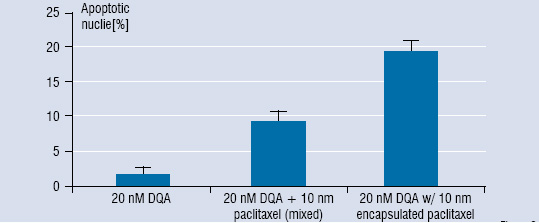
Figure 2: Activity of 10 nM paclitaxel encapsulated in DQAsomes under identical incubation conditions
Salnikov et al. employed calibrated gold nanoparticles (AuNPs) of different sizes to probe the permeability of the mitochondrial outer membrane. The authors using such nanotechnological approaches, were able to assess the physical diameter of the VDAC most likely to be between 3 and 6 nm. The investigators incubated rat permeabilised ventricular cells as well as isolated mitochondria under different conditions with 3 nm and 6 nm AuNP's, respectively.
They found that while the outer mitochondrial membrane was impermeable to 6 nm AuNPS in the absence of permeability transition, the smaller 3 nm AuNPs were able to enter mitochondria even in the presence of Cyclosporin A, which is known to prevent mitochondrial permeability transition.
Ju-Nam et al. recently introduced a strategy for attaching mitochondriotropic ligands to the surface of AuNPs. With their new approach, the authors have been able to prepare 5-10 nm sized AuNPs with surface-attached triphenylphosphonium residues, the mitochondria-specifity of which is currently being tested in vitro.
Merging nanoscience and nanotechnology with mitochondrial medicine will create a large variety of tools and methods for analysing and manipulating mitochondrial functions. Considerable progress should be seen in using mitochondrial nanomedicine for the treatment of common diseases such as cancer, diabetes and neurodegeneration as well as for alleviating symptoms associated with ageing.
Increased understanding of malfunctions present in mitochondrial diseases will lead to more efficient diagnosis and eventually to the tailored design of highly efficient mitochondrial therapeutics.
References:
1. D. C. Chan, Mitochondrial fusion and fission in mammals, Annu Rev Cell Dev Biol 22 (2006) 79-99.
2. R. Medda, S. Jakobs, S. W. Hell, and J. Bewersdorf, 4Pi microscopy of quantum dot-labeled cellular structures, J Struct Biol 156 (2006) 517-523.
3. R. K. Naviaux, Developing a systematic approach to the diagnosis and classification of mitochondrial disease, Mitochondrion 4 (2004) 351-361.
4. V. Weissig, S. M. Cheng, and G. G. D'Souza, Mitochondrial pharmaceutics, Mitochondrion 3 (2004) 229-244.
5. R. W. Horobin, Trapp, S., Weissig, V., Mitochondriotropics: A review of their mode of action, and their application fro drug and DNA delivery to mammalian mitochondria, J Control Release 121 (2007) 125-136.
6. V. Weissig, Boddapati, S.V., Jabre, L., D'Souza, G.G.M., Mitochondria-specific nanotechnology, Nanomedicine 2 (2007) 275-285.
7. V. Weissig, J. Lasch, G. Erdos, H. W. Meyer, T. C. Rowe, and J. Hughes, DQAsomes: a novel potential drug and gene delivery system made from Dequalinium, Pharm Res 15 (1998) 334-337.
8. G. G. D'Souza, S. V. Boddapati, and V. Weissig, Gene therapy of the other genome: the challenges of treating mitochondrial DNA defects, Pharm Res 24 (2007) 228-238.
9. V. Weissig, S. V. Boddapati, S. M. Cheng, and G. G. D'Souza, Liposomes and liposome-like vesicles for drug and DNA delivery to mitochondria, J Liposome Res 16 (2006) 249-264.
10. S. V. Boddapati, P. Tongcharoensirikul, R. N. Hanson, G. G. D'Souza, V. P. Torchilin, and V. Weissig, Mitochondriotropic liposomes, J Liposome Res 15 (2005) 49-58.
11. V. Salnikov, Y. O. Lukyanenko, C. A. Frederick, W. J. Lederer, and V. Lukyanenko, Probing the outer mitochondrial membrane in cardiac mitochondria with nanoparticles, Biophys J 92 (2007) 1058-1071.
12. Y. Ju-Nam, N. Bricklebank, D. W. Allen, P. H. Gardiner, M. E. Light, and M. B. Hursthouse, Phosphonioalkylthiosulfate zwitterions--new masked thiol ligands for the formation of cationic functionalised gold nanoparticles, Org Biomol Chem 4 (2006) 4345-4351.