Where the pharmaceutical industry is being unable to successfully translate core models of drug discovery from theory to practice, the two-pronged approach of Evolva Biotech in building a diversified and risk balanced compound pipeline stands as a case in overcoming the hurdles faced by the industry in the R&D paradigm.
The pharmaceutical industry has major, long standing, problems with its R&D productivity. Its core model to discover and develop drugs has not changed in the last 30 years. The problem is that the pharmaceutical industry is actually not very innovative in translating invention and discovery from academics into commercial space. The failure of the pharmaceutical industry to develop a cost-effective R&D paradigm is reducing the choice of approved drugs available to society. The only major innovation in this period (the use of recombinant technologies to make proteins in the 1970s) occurred outside the pharma industry and is only now becoming widely adopted by Big Pharma. Because of the lack of productivity, most companies are failing to launch new drugs at their desired rate and in recent years the Big Pharma has not delivered good returns.
More companies are adopting "search and develop" strategies instead of intensifying research innovation and are thereby reducing research spending. However, there is still no evidence that pharma R&D productivity is improving. In some areas, this is leading to innovation from governments, charities and other bodies in how drug development is funded. For startup drug discovery set-ups, this means that public-private partnerships are becoming increasingly important sources of funds / revenues. The conservatism of the pharmaceutical industry has also meant that it has failed to consider that the discipline of molecular genetics can impact not only large molecules but small molecules as well. Small molecule drugs, account for approximately 90% of the industry revenues. Whilst this figure may come down, it is not likely to radically change in the near future, given the advantages of small molecules. They can penetrate cells, can be made orally available and they are also relatively cheap to manufacture. Instead the industry has remained focussed on synthetic organic chemistry as the mainstay of how compounds are made-an approach with major constraints, including throughputs (a good medicinal chemist can make on an average 10 compounds a month), creativity and chemical diversity (only a fraction of the natural products can be effectively synthesised in a scalable manner).
In order to improve both the innovativeness of drugs, and research productivity it became obvious that a new approach was needed. This came about with Evolva's Genetic Chmistry and decentralised multi-polar business model which overcomes some of the current limitations.
Evolva Biotech adopts a two-pronged approach to meet the demands of the industry and other stake-holders-in building a diversified and risk balanced compound pipeline. 'Genetic Chemistry' is a proprietary technology owned by the Swiss-based multinational drug discovery company.
A technological standpoint of the cutting-edge genetic chemistry includes its directed evolution-based WatchMaker platform and networking with clusters of excellence (of public-private partnerships) to generate win-win collaborations. At an underlying level, all of the problems afflicting natural products derive from one issue-natural compounds are the result of natural selection, and natural selection does not optimise compounds to prepare human pharmaceuticals. Evolva's technology platform attempts to overcome this hurdle and aims to convert yeast cells into 'miniature' drug factories.
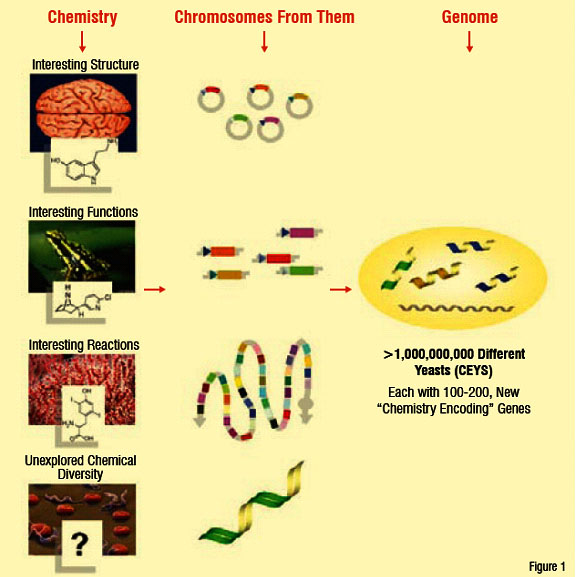
Evolva's genetic chemistry begins by sourcing genes, that produce therapeutic small molecules from species with prior established activity in a chosen disease area and cloning them into baker's yeast after stitching them together. The idea is to confer the power of nature's combinatorial chemistry capabilities onto these yeast cells grown under controlled conditions. They often have structures and properties that are unavailable to even the best synthetic chemists.
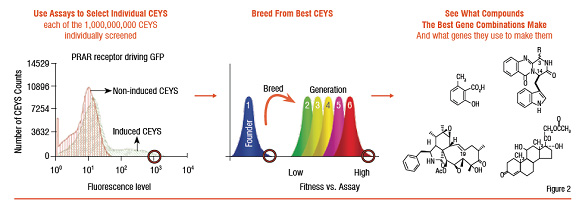
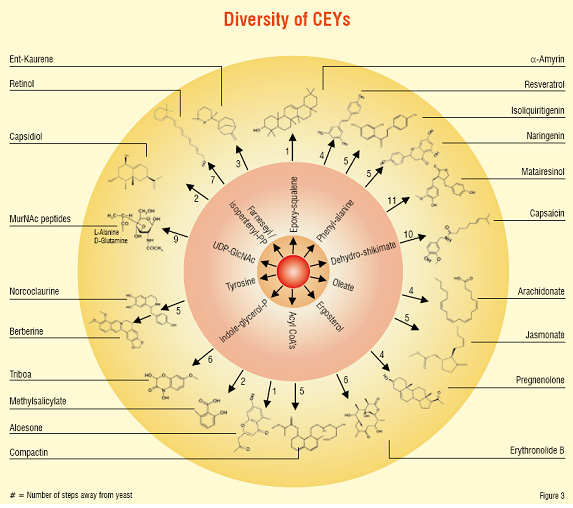
Yeasts that get "transformed" with the genes to produce combinations of small molecules are termed as "Compound Enhanced" or simply called 'CEY'. These CEYs can now produce combinations of natural small molecules (Figure 1). If this yeast is transformed again with the genes relevant to a biological target of interest, then the single yeast can produce small molecules against the biological target upon a trigger. Similar to what a medicinal chemist does in a laboratory. The "successful" yeasts are selected through a high-throughput flow cytometry system, at about 10,000 cells per second (Figure 2). This is an example of an innovative technology that allows access to a diverse chemical space (Figure 3). Having the potential to yield New Chemical Entities (NCE) for a chosen target (in the disease area) that are likely to be 'safe' (on the simple assumption that the yeast has survived) and with a potential IP. Known molecules / drugs with "poor" drug-like characteristics can also be passed through this system to improve their drug qualities like solubility and safety. This opens new avenues of in-licensing molecules for the system and out-licensing molecules from the system.
Discovering a new drug is all about maximising the probability of success and having an effective risk mitigation and management strategy. Since genetic chemistry depends on 'biodiversity', the Indian site can play a decisive role in this region. India has a traditional chemistry strength which translates to having good chemists (analytical) to assist with the novel scaffolds that come out of the platform technology.
Evolva's model has thus been created around talent pooling across various related domains and sites thereby creating a seamless work flow. This again is completely a different approach to the one site approach followed by many companies.
Translating a technology platform into drugs in clinic requires not just financial support but a dedicated and quality team with sufficient domain expertise and many 'win-win' partnerships with an objective 'ecosystem' around it. "Seed-funding" Venture Capitalists (VC) play an important role in such start-ups. Evolva has been funded by Novartis, Astellas and many others in Europe and it also required funding and collaborative initiatives to establish the roots in India. However in India, traditionally a promoter-driven industrial scene is predominant that doesn't have the appetite for high risk ventures like discovering new drugs; also, not many early stage venture capitalists were present in this area. This was the scene until recently. Andhra Pradesh Industrial Development Corporation's (APIDC) venture arm, Venture East came up with India's first dedicated biotechnology venture fund corpus which was created with the main focus on early stage companies.
One of the key benefits that these early stage VCs confer upon their portfolio companies is the high level of networking and important business contacts in that region. It was just natural that Evolva and Venture East came together in India. Evolva established operations inside the Indian Institute of Chemical Technology (IICT), a premier Council of Scientific and Industrial Research (CSIR) institution of the Government of India. Through this collaboration, Evolva could access the country's premier chemistry resources for analoging, scale up and, synthesis while IICT benefits from the biology expertise of Evolva. This association led to further collaborations. Evolva has forged a nation-wide collaboration with National Chemical Laboratory (NCL) Pune, Indian Institute of Chemical Biology (IICB) and other CSIR institutes to harness the wealth of chemistry and biology.
Though Evolva's genetic chemistry promises to deliver innovative small molecules, yet other avenues for sourcing small molecules through a fast tracking concept was also needed to hedge risks. The multi-polar model of Evolva adopts four diverse routes to source and acquire natural small molecules for the discovery pipeline in a cost-effective mode utilising the collaborations. Molecules sourced from each of the approaches would enter various stages of drug discovery cycle directly. Each approach requires a collaborative partner to succeed and primarily designed to avoid "late-stage attrition" or to encourage "early failure" of drug candidates.
The first approach is accessing natural product libraries through collaborations with various academic institutions within India and abroad subject to contract terms. These libraries with vast chemical diversity space are a valuable resource for screening on high content assays. While those molecules with prior in vitro activity would be subject to secondary confirmatory ex vivo tests, other molecules with unknown activity (in the disease areas of interest to the company) would be subjected to primary ex vivo assays. The third-party institutions from whom sourcing was done, is kept informed about movement of molecules at every stage through regular communication channels. A few hundred molecules with expected activity profile are progressed to the next stage.
The second approach is rational drug design. In each of the chosen disease areas, a validated drug target with crystal structure availability is chosen for the in silico modelling approaches. Several thousand diverse set of compounds from commercially available natural product libraries and catalogues can be chosen and screened against the targets in silico-by a carefully chosen bioinformatics collaborator. India's established IT skills and bioinformatics prowess presents a variety of options of collaborating either with academia or industry or both. Top scoring molecules are then purchased and subject to screenings. If they are found to perform the desired activity, they can be passed onto the next stage.
The other two approaches of Evolva include public-private partnerships and conventional in-licensing models. Networking with clusters of excellence-both within and outside India considered a key to future success of drug discovery start-ups. Also bridging industry-academia gap is an important component to increase odds of success-as witnessed by activities in the discovery pipeline. Most of the premier institutions of the Government of India have research programmes but, they are not going all the way to drug discovery. In most of the cases the research culminates into a preliminary pre-clinical work. Evolva has successfully managed several collaborations with leading CSIR research institutions of the Government with a very clear road map of progressing on these compounds with appropriate milestones. The costs for development are shared under a mutual agreement and in-licensed wherever possible. In a few cases, where there is more conclusive pre-clinical evidence, Evolva tries to have an outright in-incensing deal early in the discovery stage.
Evolva also leverages the India advantage in the development phase where there are various Contract Research Organisations (CROs) in each segment in animal pharmacokinetic and efficacy studies, GMP scale-up operations, GLP compliant animal trials and even all phases of clinical trials that can be conducted by the Contract Research and Manufacturing Services (CRAMS) companies. Indian CRAMS not only offer cost advantages but also the value additions which are again key to maximising chances of success.
In short, Evolva has used a very pragmatic, aggressive and international business model to bring out drugs with drug development costs possibly below the sub-billion dollar level. This trend setting decentralised model is rather unique for a small company. Nevertheless, this is likely to be "different" business model which leverages the strength of cost arbitrage and value of human capital from across multiple sites.
Evolva is a Swiss-based drug delivery company whose WatchMaker technology relies on assembling genes and pathways in the development of small molecules to drug targets. Evolva established an India operation less than two years ago with the support of India's CSIR, IICT and various investors including APIDCVCL pathways