The use of multi-functional nanosystems affords convergence of technologies for simultaneous or sequential target-specific delivery of multiple drugs or by combining drugs with different energy modalities.
In the context of biomedical imaging and therapeutic applications, nanosystems are defined as particles of less than 1000 nanometers in diameter that can overcome biological barriers and provide efficient delivery and have unique properties.
The application of nanotechnology for disease prevention, diagnosis and treatment is receiving significant attention over the last several years. Furthermore, inclusion of image contrast enhancers with drugs can serve an important function as monitors of therapeutic efficacy. Other such combinations are also possible and can have significant clinical benefits, especially in cancer. Overall, multi-functional nanosystems offer an exciting and fruitful area of research with tremendous opportunity for clinical translation.
"Nano" is the most widely used keyword today in fields such as telecommunication, electronics, clean energy, transportation, consumer goods and in biomedical technologies. Currently, the US government is investing over US$ 1 billion a year in funding for nanotechnology research, while other global investment sums to US$ 4 billion a year. In contrast, private funding for nanotechnology research and development is minimal with a substantial portion being used to fund nano-biotech projects.
Nanomedicine is a new interdisciplinary paradigm emerging from the timely convergence of two parallel recent developments. The tremendous advancements in genetic engineering and molecular biology has led to molecular basis of diseases and nanotechnology which offer a powerful means to control molecular interactions. Nanomedicine can significantly affect millions of individuals around the world with acute and chronic diseases including cancer, cardiovascular disease and infectious diseases.
According to the 2005 Nanomarket Report, the nanomedicine market is estimated to generate about US$ 1.7 billion in revenues by 2009, and this is expected to increase to US$ 4.8 billion by the year 2012. Based on these encouraging financial forecasts, developments in nanomedicine have the potential to become an essential driving force that can propel the already high-technology based pharmaceutical, biotechnology and medical device industries to new heights.
Examples of nanotechnology applied in pharmaceutical product development include organic nano-platforms such as polymeric, lipid (e.g. liposomes, nanoemulsions and solid-lipid nanoparticles), self-assembling structures and dendrimers as well as certain inorganic nano-platforms including metal (e.g. gold and silver) and silica-based nanostructures. These nanosystems are designed to provide an optimised formulation for oral or systemic administration, protect the entrapped payload from degradation, allow greater fraction of the dose to be available at the disease site and reduce the rapid clearance of the drug from the body.
Surface modification of these nanosystems can afford passive or active targeted delivery of the payload to the disease site in the body. Doxil? (doxorubicin in long-circulating liposomes) and Abraxane? (paclitaxel in nanoparticulate albumin) are two illustrative examples of nano-therapeutics approved in the US by the Food and Drug Administration. Other nano-platform based drug delivery systems, including micellar and dendrimer-based are undergoing clinical trials in the US and other parts of the world.
Although there are several clear advantages in the use of nanosystems for targeted imaging and delivery applications, the number of clinically approved products in the US is fairly limited. There are several major constraints in clinical translation of nanotechnology-based pharmaceutical products. Due to the inherent complexity of some of the nanosystems, large-scale manufacturing under Good Manufacturing Practices guidelines and appropriate quality control can be major factors limiting development efforts. In addition, the lack of inherent toxicity of the nanocarrier system has to be clearly shown in preclinical and clinical studies.
Certain organic and inorganic nanomaterials, such as fullerenes, carbon nanotubes silica nanoparticles, and quantum dots have excellent properties, but their systemic distribution and clearance profiles, tissue and cellular interactions and associated toxicity, especially upon chronic in vivo administration, have not been clearly addressed. Additionally, since the biodistribution of nano-encapsulated drug will be quite different from that of the free drug, a comprehensive pre-clinical dose escalating toxicity studies in multiple animal species of the final product as well as certain components is required by the regulatory agency. The associated complexities and higher cost of these studies has profound impact on clinical development. These concerns become even more pronounced with complex nanocarriers having multiple therapeutic payloads, and additional targeting and imaging functionalities.
In addition to the first generation nanosystems with very useful properties, such as prolonged circulation in blood, passive or active target specificity and increased cell penetration, opportunity exists to develop multi-functional nanosystems for potentially combining various features for additive or synergistic effects (Figure 1).
For instance, the use of nano-materials that respond to physiological stimuli such as pH, temperature, or redox status, can also allow for even greater selectivity in delivery to the target tissue, upon cellular internalisation, or even in specific subcellular organelle. Additionally, multi-functional nanosystems can take advantage of a single carrier platform to incorporate multiple therapeutic agents for simultaneous or sequential delivery, combining drugs with energy (e.g. heat, light and sound) for enhanced therapeutic effect and combining drug(s) with imaging agent to monitor the therapeutic efficacy in real time.
Nanosystems that can encapsulate multiple therapeutic modalities, such as drugs and photosensitisers, could also provide an interesting multi-pronged synergistic therapy based on the biological activity of the drug and light-activated photosensitiser. Another promising avenue involves the use of combination chemo and thermal therapies. There is substantial data including animal and clinical studies taking advantage of complimentary interactions between hyperthermia, thermal ablation and intravenously administered liposomes containing doxorubicin.
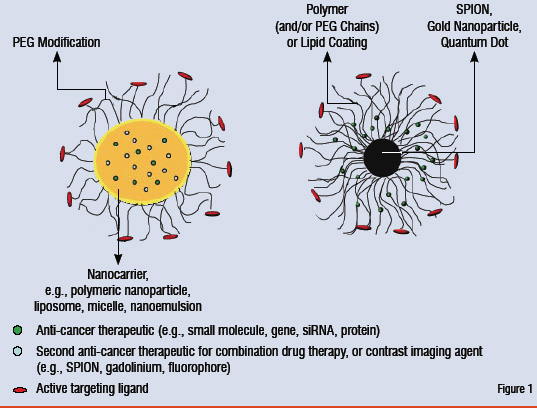
Figure1: Multi-functional nanosystems for targeted delivery of imaging and therapeutic molecules
The road ahead
There is an unprecedented opportunity for nanotechnology in biomedical and pharmaceutical applications. Through parallel advances in understanding molecular
Nanosystems for Cancer Therapy
Over the past several years, our group has developed a variety of polymeric nanoparticles for tumour drug delivery all leading to an enhanced in vivo therapeutic efficacy. Some examples of these include the delivery of tamoxifen and paclitaxel in poly(ethylene oxide)-modified poly(epsilon-caprolactone) (PEO-PCL) nanoparticles in breast cancer model, the delivery of paclitaxel in PEO-modified PCL and PEO-modified poly(beta-aminoester) (PbAE) nanoparticles in ovarian cancer and even the delivery of reporter (GFP and b-galactosidase encoding) and therapeutic genes (i.e. sFlt-1 or VEGF-R1 encoding) in murine and human tumor xenograft models. This versatility of polymer platforms allows for fine tuning of the drug delivery formulation to meet specific advantages. For example, the composition can be tuned to provide precise drug capture or release in response to environmental triggers. Alternatively, the composition can even be optimised to allow for inclusion of multi-functional properties, such as a combination of therapeutics, targeting and / or imaging modalities, all within one nanoparticle platform.
Due to overwhelming evidence of multi-drug resistance development with single therapeutic approach, diseases such as cancer and certain infections are treated by using a "cocktail" therapeutic approach. Multi-functional nanosystems have been designed to encapsulate multiple therapeutic agents in a single delivery system for either sequential or simultaneous delivery. For instance, poly(ethylene glycol) (PEO)-modified PbAE / poly (D, L-lactice-co-glycolide) (PbAE / PLGA) blend nanoparticles have been utilised for sequential delivery of paclitaxel and ceramide in multi-drug resistant (MDR) tumor models. In this system, paclitaxel is encapsulated in pH-sensitive PbAE matrix, while the apoptotic second messenger, ceramide, is encapsulated in the PLGA matrix. When administered to MDR solid tumor or upon cellular internalisation, paclitaxel is rapidly released from PbAE matrix due to drop in pH from 7.4 to less than 6.5, while ceramide is slowly released from the PLGA matrix by diffusion or degradation of the polymer matrix. Additionally, Sengupta, et al. have utilised a "nanocell" concept designed to deliver both anti-angiogenic (i.e. combretastatin A4) and cytotoxic (i.e. doxorubicin) drug in a system made with phospholipid-coated PLGA nanoparticles. The anti-angiogenic agent combretastatin A4 was entrapped in the phospholipids layer, while doxorubicin was conjugated with PLGA for slow release. Relative to all the controls, the nanocell deliver system provided the most enhanced efficacy with less toxicity in Lewis lung carcinoma and B16 melanoma murine tumor models.
Acknowledgements
Our research effort has received funding from the Alliance in Cancer Nanotechnology of the U.S. National Cancer Institute, National Institutes of Health. We also appreciate the collaborative opportunities with many academic and industrial scientists in the Boston area, and especially with Professor Robert Langer at MIT, Professor Vladimir Torchilin at Northeastern University, Dr Michael Seiden at Massachusetts General Hospital and Dr Takeshi Sano at Beth Israel Deaconess Medical Center.
basis of diseases and developments in sophisticated nano-engineering strategies, there is significant optimism in development novel nanosystems for prevention, diagnosis, and treatment of diseases. In drug and gene delivery area, multi-functional nanosystems can fill the critical need to overcome adverse effects through target-specific delivery and intracellular localisation, opportunity to combine different therapeutic modalities, overcome multidrug resistance, and overall improve therapeutic outcomes. Through active collaborations between basic and applied scientists and engineers and clinical practitioners, we foresee a great promise for multi-functional nanosystems overcoming many of the challenges in contemporary molecular medicine.
References:
1. NNI. Nanotechnology. [cited; Available from: http://www.nano.gov/.]
2. Maynard, A.D., Nanotechnology: the next big thing, or much ado about nothing? Ann Occup Hyg, 2007. 51(1): p. 1-12.
3. NNI. Nanomedicine. [cited; Available from: http://nihroadmap.nih.gov/nanomedicine/index.asp.]
4. Zhang, L., et al., Nanoparticles in medicine: Therapeutic applications and developments. Clinical Pharmacology & Therapeutics, 2007. doi: 10.1038/sj.clpt.6100400.
5. Devalapally, H., A. Chakilam, and M.M. Amiji, Role of nanotechnology in pharmaceutical product development. J Pharm Sci, 2007. 96(10): p. 2547-65.
6. Nanomarkets, Impact of nanotechnology in drug delivery: Global developments, Market Analysis and Future Prospects. 2005.
7. Wilson, R.F., Nanotechnology: the challenge of regulating known unknowns. J Law Med Ethics, 2006. 34(4): p. 704-13.
8. van Vlerken, L.E. and M.M. Amiji, Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin Drug Deliv, 2006. 3(2): p. 205-16.
9. Torchilin, V.P., Multifunctional nanocarriers. Adv Drug Deliv Rev, 2006. 58(14): p. 1532-55.
10. Shenoy, D.B. and M.M. Amiji, Poly(ethylene oxide)-modified poly(epsilon-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. Int J Pharm, 2005. 293(1-2): p. 261-70.
11. Devalapally, H., et al., Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 3. Therapeutic efficacy and safety studies in ovarian cancer xenograft model. Cancer Chemother Pharmacol, 2007. 59(4): p. 477-84.
12. Shenoy, D., et al., Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 2. In vivo distribution and tumor localization studies. Pharm Res, 2005. 22(12): p. 2107-14.
13. Kaul, G. and M. Amiji, Tumor-targeted gene delivery using poly(ethylene glycol)-modified gelatin nanoparticles: in vitro and in vivo studies. Pharm Res, 2005. 22(6): p. 951-61.
14. Kommareddy, S. and M. Amiji, Antiangiogenic gene therapy with systemically administered sFlt-1 plasmid DNA in engineered gelatin-based nanovectors. Cancer Gene Ther, 2007. 14(5): p. 488-98.
15. Sengupta, S., et al., Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature, 2005. 436(7050): p. 568-72.
16. Hauck, M.L., et al., Phase I trial of doxorubicin-containing low temperature sensitive liposomes in spontaneous canine tumors. Clin Cancer Res, 2006. 12(13): p. 4004-10.
17. Kong, G., et al., Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res, 2000. 60(24): p. 6950-7.
18. Needham, D., et al., A new temperature-sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft model. Cancer Res, 2000. 60(5): p. 1197-201.
19. Needham, D. and M.W. Dewhirst, The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv Drug Deliv Rev, 2001. 53(3): p. 285-305.
20. Ponce, A.M., et al., Hyperthermia mediated liposomal drug delivery. Int J Hyperthermia, 2006. 22(3): p. 205-13.
21. Viglianti, B.L., et al., In vivo monitoring of tissue pharmacokinetics of liposome/drug using MRI: illustration of targeted delivery. Magn Reson Med, 2004. 51(6): p. 1153-62.