Nanotechnology offers an innovative platform for the development of multivalent therapeutic systems with advanced pharmaceutical properties. Nano-particulate drug delivery systems have gained a lot of attention due to their characteristic features such as sustained drug release, targeting capability along with reduced toxicity profiles. In recent years, this potential has translated into commercially available products for the management of various diseases.

The concept of nanotechnology was first described by Nobel laureate Richard Feynman in a popular lecture he gave entitled ‘There’s Plenty of Room at the Bottom’ on 29 December, 1959. The initial applications conceived through nanotechnology were limited to atomic scale physics and information storage. Today, nanotechnology has reached far greater heights and is applied in diverse fields such as high-performance capacitors and batteries, optical imaging / contrast agents and most importantly, as drug delivery systems for the clinical management of various diseases such as cancer and HIV. The advancement of science and technology has made it possible for nanotechnology-based products to be commercially available; and since the early 1990’s, many nano-pharmaceuticals have gained US Food & Drug Administration (FDA) approval. Modern approaches combined with advances in genetic and molecular biology can eventually pave the way for personalised medicine and therapies in the future.
Nanotechnology refers to the applications of nanoscale materials to achieve innovation. Nanomaterials offer unique properties and characteristics, which are completely different from bulk matter. They range from nanoparticles which are simple and are about 1-100 nm in dimension to more complex structures such as dendrimers, solid lipid nanoparticles, core-shell nanoparticles, and conjugated drug delivery systems. The most fundamental aspect of nanomaterials is their nano-dimension. Its associated implications on the overall drug profile can be exploited in the way of new formulations and delivery systems. Their unique and intrinsic properties can be directly attributed to the increase in surface to volume ratio at the nanoscale. Surface functionalisation through suitable chemical moieties and specific markers helps in targeted drug delivery and masking from immune system. Their bio-compatibility can be improved; even anatomically reserved sites such as the blood-brain-barrier can be accessed. (Figure 1 – Need for nanotechnology)
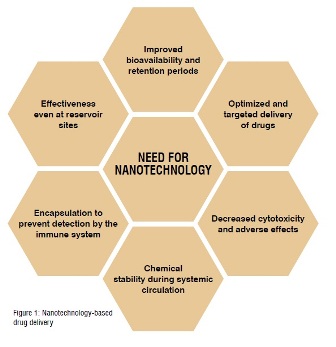
Nanotechnology-based drug delivery systems offer superior pharmaceutical properties when compared to conventional dosage forms, mainly through entrapment of the drug into a nano-system. The important pharmaceutical parameters such as bioavailability and bio-distribution can be regulated and limitations can be overcome through nano-pharmaceuticals. Increased circulation times and sustained release are also an accompanying feature of nanotechnology-based drug delivery systems. The nano-dimensions provide increased surface area and, as a consequence, bioavailability and drug permeability increases, achieving the desired therapeutic effect.
The pharmacokinetic and pharmacodynamic profile of a drug can be regulated through numerous approaches and one of the ways is through the design and synthesis of nanotechnology-based drug delivery systems. Targeted drug delivery and access to reservoir sites has been a long-standing hurdle to scientists and pharmacists in drug research and development. Specific chemical moieties and functional groups can be used as probes for targeting particular sites and receptors. This has been proved to be successful in the case of cancers, where there needs to be a distinction between normal tissue and tumour affected region. Through ligand based targeting mechanisms, intracellular drug accumulation and uptake by the targeted tissue can thus be regulated. Advances such as the development of pH- and thermo-sensitive polymers have enriched the targeting potential of nanotechnology. Therapeutic efficacy at the terminal site of action has been an important pharmaceutical parameter of drug molecules and anatomically privileged reservoir sites can be accessed and effectively treated through nanotechnology-based drug delivery.
Adverse effects and dose-related toxicities of many therapeutic agents may prove to be a hindrance in the effective management of diseases. However, by employing nanotechnology-based interventions, these issues can be easily overcome. Nano-particulate drug delivery systems have markedly improved permeability characteristics along with increased intracellular drug accumulation and hence, lower doses with reduced side-effects are possible through nano-based approaches. Importantly, reduced toxicity profile of drugs can be achieved while retaining therapeutic efficacy.
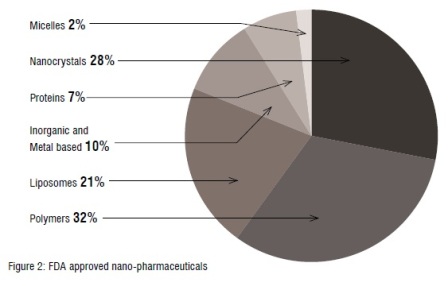
Nanotechnology-based drug delivery of pharmaceutical drugs has been attempted with varying degrees of success. The early objectives and benefits perceived through nanotechnology were limited to improved efficacy, PK-PD parameters and safety profile. The progress in science and technology has helped in the overall growth and evolution of nanotechnology-based drug delivery. Complex designs and mechanisms of fabrication are a reality and such nano-carriers conjugated with drug molecules are proving to be gamechangers in the pharmaceutical industry. Majority of FDA approved nano-drugs are liposomal or polymeric in nature or synthesized as nanocrystals and are currently available.
During the last three decades, close to 50 nano-based pharmaceutical drugs have gained FDA approval and marketed commercially. Also, more than 70 nanopharmaceuticals are undergoing clinical trials, with many of them initiated in 2014 or later (Bobo et al., 2016). FDA approvals of nano-drugs peaked between 2001 and 2005, followed by a substantial drop after 2006, possibly due to lower investment caused by the economic crisis of 2008. There has been a gradual rise in the number of nano-drugs that have received investigational new drug (IND) approval from the FDA to undergo clinical trials since 2007. The years from 2013 to 2015 had the highest number of nanoformulations entering clinical trials and this suggests an increase in the availability of FDA-approved nano-drugs in the future (Ventola, 2017).
Conventional methods and mechanisms of drug delivery have their own limitations and drawbacks such as limited efficacy and to an extent, a lack of selectivity. Many pharmaceutical drugs have variable absorption rate (oral administration) and also the digestive enzymes and acidic environment can break down some of the drugs before they enter blood circulation and become bio-available. Lack of specificity and selectivity of drug molecules arises due to poor bio-distribution and as a result, other tissues and organs can be damaged due to their toxicity.
Nanotechnology-based drug delivery approaches are ideal for the clinical management and treatment of various diseases. Such systems have primarily shown to increase the circulation and retention times, thereby improving the half-life and bio-availability of drugs and ultimately leading to their greater efficacy. Also, as most drugs are hydrophobic in nature, their solubility can be enhanced through nano-based approaches. Sustained, targeted, and controlled release of drugs can be achieved using nanotechnology. Many such nanotechnology-based drug delivery methods and systems have been approved for use in the management of various diseases.
Cancer is the uncontrolled growth of abnormal cells with the potential to spread to other parts of the body. According to the World Cancer Report, in 2018, the number of new cancer cases was estimated to be 1.16 million and around 784,800 cancer deaths in India. Treatment options for cancer include chemotherapy, radiation therapy and surgery. Primary treatment involves the administration of chemotherapeutic agents and most cancer drugs have dose limiting toxicities accompanied with severe adverse effects and can increase the risk of secondary cancers, especially due to alkylating agents.
Most nanotechnology-based drugs were granted approval by the FDA based on their lower toxicity compared with conventional formulations and many more are in clinical development and trials. Doxil (Janssen) was the first nanotechnology-based drug to be granted FDA approval in 1995. It is a liposomal formulation of doxorubicin hydrochloride. The traditional dosage form was reported for its cardiotoxicity and the nano-drug (Doxil) was approved on the basis of significantly lower adverse effects. It has been in use for more than twenty years and is still widely used for the treatment of Kaposi’s sarcoma, ovarian, breast and other cancers (Ventola, 2017).
Emend (Merck) is the nano-crystalline form of the anti-emetic drug aprepitant and was granted FDA approval in 2003. It is mainly used to prevent chemotherapy associated nausea and vomiting. The absorption of aprepitant occurs in the upper gastro-intestinal (GI) tract and aprepitant is insoluble in water. Emend has its main advantages in increased absorption rates in the GI tract and improved bio-availability.
Drug molecules can be conjugated to biomolecules, polymers and nanoparticles to significantly increase their sitespecificity and subsequently, reducing the adverse effects. Onivyde (Merrimack Pharmaceuticals) is another nanotechnology- based liposomal formulation of irinotecan and is mainly used in the treatment of metastatic pancreatic cancer and received FDA approval in 2015. The main advantages are increased delivery to the tumour site and reduced systemic toxicity.
Abraxane (Celgene) is a nanotechnology-based drug formulation containing paclitaxel bound to albumin NPs and was approved by the FDA in 2005 for metastatic breast cancer. Abraxane is significantly more tolerable than conventional dosage form of paclitaxel and can be administered at a much higher dose, giving rise to increased efficacy.
Thus, the dose-limiting toxicities associated with conventional chemotherapeutic and anticancer agents can be overcome through nanotechnology. Various cancer drugs are essentially hydrophobic and poorly soluble in aqueous solutions. Therefore, the need arises for solubilising agents which increase the overall toxicity and often these drugs require dose reduction to reduce systemic toxicity. To overcome the toxicity and solubility related issues, nanotechnology-based drug delivery offers an innovative and promising means (Farjadian et al., 2019).
The human immunodeficiency virus (HIV) primarily targets the CD4+T cells and eventually weakens the immune system, leading to the Acquired Immunodeficiency Syndrome (AIDS). Since 1980’s, it has gradually become a devastating pandemic and caused significant burden to the global health scenario. There is no cure or vaccine yet and only suppressive therapeutic interventions are currently available, known as HAART (Highly Active Antiretroviral Thearpy). Also, the dosing regimen of HAART is an important criteria for its success. Nanotechnology based drug delivery approaches have been employed to circumvent these plaguing issues.
The antiviral efficacy of HAART therapy is limited by the distinct pharmacokinetic profiles of partner therapeutics which lead to varying bio-distribution and associated adverse effects. Li and colleagues developed a new cocktail-like drug delivery vehicle using biodegradable polymeric nanoparticles which contained a non-nucleoside reverse transcriptase inhibitor (NNRTI) and another HIV-1 fusion inhibitor conjugated to the surface. These nano-vehicles showed increased cellular uptake and circulation times and potent in-vitro activity against HIV (Li et al., 2016).
Kumar et al., attempted a triple drug combination of first line HIV drugs which were loaded into lactoferrin nanoparticles. The combination of zidovudine, efavirenz and lamivudine in lactoferrin nanoparticles exhibited greatly improved pharmaceutical properties with lesser tissue-related inflammation. The in-vitro activity against HIV virus was also appreciable and better when compared to the free drug combination (Kumar et al., 2017).
The herpes simplex virus (HSV) causes persistent HSV infections, which are characterised by the lesions or sores in the oro-labial region or the genitals. This infection if left untreated, can lead to severe conditions such as herpes keratitis or herpes meningoencephalitis. Nucleoside analogues such as acyclovir are generally prescribed to suppress the symptoms and offer relief. One main factor affecting the usage of acyclovir is its limited oral bio-availability and efficacy, due to its low permeability across the GI tract. To overcome this issue, Al-Dhubiab and co-workers used acyclovir loaded into nano-spheres, which were then incorporated into a film for buccal administration and such a nanotechnology-based drug delivery system showed significantly increased bio-availability, circulation times and greater efficacy (Al-Dhubiab et al., 2015).
The impact of nanotechnology-based drug delivery approaches and systems has transcended beyond the pharmaceutical industry and is currently felt in many more clinical applications such as imaging, diagnosis and treatment of various diseases. They have numerous advantages when compared with conventional drug delivery strategies. Nano-based systems are comparable in size with biomolecules and hence, their interactions can be tailor-made to suit clinical applications. Lately, a growing number of researchers and academicians are shifting their focus from simple nanoparticles to more evolved and complex systems.
Currently, most of the approved nanobased drugs are simple nano-formulations consisting of previously approved FDA drugs. The number of nanotechnologybased drugs entering clinical trials has increased in the recent years and the trend of many more nano-drugs gaining FDA approval is bound to increase. The translational potential of nanotechnology-based drug delivery will continue to grow and prove to be a game-changer for academics, researchers, clinicians and the overall pharmaceutical industry at large.
With progression of newer research and knowledge gained from the complex interactions between host-organ systems and nanomaterials, the future of more innovative nanotechnology-based pharmaceutical products being developed and gaining regulatory agencies’ approval also increases. The promise of personalised medicine can be realised through such nano-based multivalent therapeutic interventions and the ever-elusive cure for many complex and rare diseases can be found. However, for wider applications and usage of nanotechnology in the pharma industry, further research involving in-vivo studies and clinical trials are needed to understand their toxicity and long-term benefits.
REFERENCES:
1. Al-Dhubiab, B. E., Nair, A. B., Kumria, R., Attimarad, M., & Harsha, S. (2015). Formulation and evaluation of nano based drug delivery system for the buccal delivery of acyclovir. Colloids and Surfaces. B, Biointerfaces, 136, 878–884. https://doi.org/10.1016/j.colsurfb.2015.10.045
2. Bobo, D., Robinson, K. J., Islam, J., Thurecht, K. J., & Corrie, S. R. (2016). Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials cto Date. Pharmaceutical Research, 33(10), 2373–2387.https://doi.org/10.1007/s11095-016-1958-5
3. Farjadian, F., Ghasemi, A., Gohari, O., Roointan, A., Karimi, M., & Hamblin, M. R. (2019). Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine (London, England), 14(1), 93–126. https://doi.org/10.2217/nnm-2018-0120
4. Kumar, P., Lakshmi, Y. S., & Kondapi, A. K. (2017). Triple Drug Combination of Zidovudine, Efavirenz and Lamivudine Loaded Lactoferrin Nanoparticles: an Effective Nano First-Line Regimen for HIV Therapy. Pharmaceutical Research, 34(2), 257–268. https://doi.org/10.1007/s11095-016-2048-4
5. Li, W., Yu, F., Wang, Q., Qi, Q., Su, S., Xie, L., Lu, L., & Jiang, S. (2016). Co-delivery of HIV-1 entry inhibitor and nonnucleoside reverse transcriptase inhibitor shuttled by nanoparticles: cocktail therapeutic strategy for antiviral therapy. AIDS (London, England), 30(6), 827–838. https://doi.org/10.1097/QAD.0000000000000971
6. Ventola, C. L. (2017). Progress in Nanomedicine: Approved and Investigational Nanodrugs. P & T : A Peer-Reviewed Journal for Formulary Management, 42 12), 742–755.