Efficient drug delivery to eye remains a major challenge for the pharmaceutical researchers worldwide due to the number of barriers offered by this sensitive organ namely, static barriers dynamic barriers and metabolic barriers. Bypassing these barriers to reach the target site is the key to optimal therapeutic effect of ocular drugs. As the anatomy and physiology of each barrier is unique the ways to bypass them should also be different. This review summarises the various challenges and newer trends in the ocular drug delivery.
As per the estimation of a Forbes analysis (2013), around US$5 billion is spent by large pharmaceutical companiesfor a single drug approval. Despite immense increase in the research expenditure and number of compounds in the development phase, the rate of approval of these compounds is fairly less due to the lack of drug efficacy. This innovation drought is also a major concern of ophthalmic care market. Though anti-VEGF therapies in wet AMD and Xalatan (latanoprost) for glaucoma have been a breakthrough discovery, yet effective and tolerable treatments are not available for a large number of ocular diseases. Expiry of patents in addition to failure of new proprietary drugs launch indicates significant impact of generics on the therapeutics market. Even though new drugs have high market potential, stringent regulatory requirements pose hurdles at late stage of clinical development. Therefore, fine-tuning and improvement of existing products is highly desired rather than the development of a whole new class of ophthalmic drugs. To achieve this, knowledge about pathophysiology of ocular diseases and limitations of current therapies is the need of hour but before that a thorough understanding about the anatomy and physiology of eye is crucial to design and develop an effective/safe dosage form. The unique environment of eye can offer major barrier to drug delivery. This review summarises various challenges and opportunities in ophthalmic drug delivery.
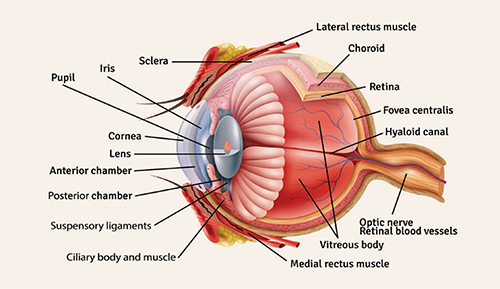
Unique ocular environment:
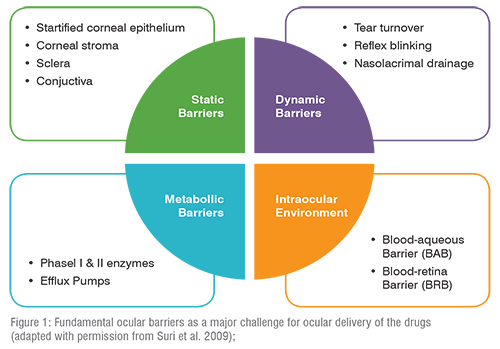
A significant challenge faced by the formulators and research scientists worldwide is bypassing the protective barriers of the eye without causing any damage to the ocular tissues. These static (biological), dynamic and metabolic barriers result in poor bioavailability of topically administered drugs (Figure 1). Also, high efficiency of the blood-retinal barrier (BRB) makes it difficult for the therapeutics to reach the posterior segment which is compromised in retinal degenerative disorders. Maintaining therapeutic drug concentration over prolonged periods in the eye is yet another challenge. Furthermore, the disorders of lacrimal secretion and treatment of lacrimal gland are still not so explored areas. The presence of melanin in uvea and retinal pigmented epithelium (RPE) also lowers the pharmacological activity of the therapeutic agent, necessitating the administration of higher amount of doses. Figure 1
Choice of route of administration:
Though topical route is a non-invasive and highly patient compliant route, nasolacrimal drainage, high tear dilution, efflux pump and the corneal barrier limits the drug bioavailability. Additionally, it is inefficient for the disorders of the posterior segment. Intravitreal route on the other hand is suitable for posterior segment drug delivery but is invasive in nature with many complications (vitreous haemorrhage, retinal detachment, and endophthalmitis). Alternative route like Periocular is favuorable for controlled drug delivery for a long period of time but presence of retinal pigment epithelium offers an obstacle. Systemic route of drug delivery has good patient acceptance but encounters the blood-ocular barriers and the need of high dosing resulting in systemic toxicity often makes it unsuitable.
Physicochemical drug properties:
Certain drug properties such as lipophilicity, molecular weight, molecular size and shape, charge, solubility, and degree of ionisation significantly influence the route and rate of permeation across the ocular membranes. Therefore, amphipathic nature of the molecule is desired for permeation through the ocular layers.
Predictive animal models:
Animal models that more accurately simulate human ocular disease gives better drug efficacy results, define model therapeutic mechanisms of action and suggest optimal target receptor in a cost-effective manner before the expensive clinical trials are carried on. Due to the lack of availability of suitable animal models, selection of drug for clinical investigations and further human disease study is often limited. A greater challenge is posed in case of diseases like Thyroid Eye Disease which is resistant to modelling in animals1.
Overlapping clinical resemblance of diseases:
It has been observed that due to superficial clinical resemblance, a number of conditions are grouped together which are a result of different etiological process and thus demand different therapeutic strategies. For example, in ocular inflammatory diseases, many forms of uveitis are ‘lumped together’ in the same clinical trial that require different outcome measures, may be due to poorly defined disease phenotype, poor understanding of pathogenesis or lack of clear diagnostic markers etc. Moreover, if the investigators increase the size of trial, due to the low ‘signal-noise’ ratio the chances to identify a successful treatment decrease1.
Prediction of Outcome measures:
Outcome measures, mostly non-invasive like visual acuity, or intraocular pressure that have good patient acceptability and early detection, are key factor for ophthalmic studies. The problem occurs when outcome measures are subjective (whether reported by the patient or the clinician), imprecise and not fully quantitative.
The isolated environment of eye gives great opportunity to treat the ocular diseases with potentially no systemic side effects. Different target strategies are thus employed by the formulation scientists and researchers globally in order to design and develop an effective and patient acceptable drug delivery system that transport the drug to the target area bypassing different ocular barriers.
Conventional ocular formulations like solutions, emulsions, suspensions, and ointments still retain their place in the ophthalmic market at large due to their improved solubility, pre-corneal residence time and ocular bioavailability of drugs. Much efforts are also being made to deliver the therapeutics to the posterior segment of the eye with these conventional carriers. However, various associated side effects such as ocular irritation, vision interference, inflammation, redness and stability issues demand further research to improve the in vivo performance of these formulations and to minimise their side effects. Cyclodextrins, permeation enhancers (surface active agents, chelating agents, bile salts) and viscosity enhancers (hydroxy ethyl cellulose, sodium carboxy methyl cellulose, hydroxypropyl methyl cellulose, polyalcohol) improve the corneal uptake, permeation, drug contact time and ocular bioavailability. Marketed ocular emulsions in the United States includes Restasise™, Refresh Endura® and AzaSite®. Also, high viscosity of Tobra Dex® suspension was improved to give (TobraDex ST®) with better formulation characteristics and in vivo pharmacokinetics along with bactericidal activity.
Despite considerable efforts being made to optimise conventional formulations, to overcome their drawbacks, research horizons are expanding in the field of nanotechnology. Novel strategies (appropriate particle size) are designed to deliver therapeutics to the target ocular tissue in both anterior and posterior segment, with low irritation, adequate bioavailability, and ocular tissue compatibility in a controlled/sustained manner. Several nanocarriers, such as nanoparticles, liposomes, nanomicelles and dendrimers have been developed for drug delivery to eye.
The dendrimer have a unique structure that increases water-solubility of drugs and enhance corneal residence time of topically administered therapeutic agents. Biocompatible vesicular systems like liposomes are widely used to deliver both hydrophilic and hydrophobic drugs to the eye but are not completely devoid of the drawbacks. Thus, liposomes have been modified by the use of polyol or coating with chitosan, in order to overcome the problems of stability (aggregation), leakage of drugs, low encapsulation efficiency and improve the residence time in the cornea. Nanomicelles (Polymeric, Surfactants and Polyionic complex micelles) are also utilised in ocular drug delivery due to the advantages like easy permeation through the ocular epithelium, minimal drug degradation, enhanced bioavailability and no irritation. Biodegradable implants like Ozurdex® and Surodex® and contact lens are also used largely to deliver drugs to the eye. Additionally, for the treatment of posterior segment diseases, non-invasive methods like microneedle, ultrasound, and iontophoresis-based ocular drug delivery systems are employed to deliver drugs to intraocular regions. Corneal permeability of atenolol, carteolol, timolol and betaxolol, has been significantly enhanced with ultrasound. Transcorneal iontophoresis of ciprofloxacin hydrochloride and gentamicin have also gained much attention. Advanced ocular iontophoresis device has been developed by EyeGate. Currently, biocompatible devices like Punctum plugs/occludes or lacrimal plugs are developed that are inserted in the tear ducts to block tear drainage. These are non-invasive and provide controlled drug release to the anterior segment of the eye. Non-biodegradable Punctum Pug Delivery System (PPDS) are made from silicone, polycaprolactum and hydroxyethyl methacrylate. Some of the examples includes SmartPlug®, Ocular TheraputixTM (Bedford, MA, USA) and Dextenxa®. Furthermore, for the delivery to posterior segment, technologies like DurasertTM, NovadurTM and I-vationTM have been investigated upon.
To overcome the problems of insufficient solubility and transport of the therapeutics, prodrug technology is utilised, for example, acyclovir dipeptide prodrugs have been synthesised in order to target the oligopeptide transporter hPEPT1 on the cornea. Similarly, expression of transporters on various ocular tissues like cornea, conjunctiva and RPE gives us an opportunity to target the therapeutics to these transporters in-turn increase their levels and therefore decrease the dose required. A more recent approach has been taken into account by Neurotech to develop an Encapsulated Cell Technology (ECT) implant that consist of genetically engineered cell lines to continuously deliver therapeutic proteins directly into the vitreous for up to two years.
Conclusion
Regardless of various challenges in ocular drug delivery, newer products are continuously entering the ophthalmology market owing to its market size. The focus should be directed towards the design and development of a noninvasive, easy to use, sustained drug release system for eye disorders in both the segments with minimum side-effects. This would be possible only if the understanding and knowledge about the complexities associated with normal and pathological conditions, anatomical and physiological barriers, apt animal modelling and drug pharmacokinetics is increased. A combination of technologies may provide successful outcomes. The persistent challenge faced by the ophthalmic care is the ageing of the eye. Increased prevalence of ocular diseases, especially posterior segment diseases in the ageing populations gives us an opportunity and motivation for further innovation. Global ophthalmic therapeutic market is growing at two-and-a-half times the rate of the overall pharmaceutical industry and is expected to grow exponentially in the near future with many commercial benefits (high revenues) of ophthalmic drugs if the research is directed in the right direction.