Regulatory awareness has led to the need for new approaches when regulating nanotechnology in the inhaled area. Opportunities as well as challenges exist. If addressed, they can open the doors for new IP to be generated along with considerations that can be incorporated in Life Cycle Management.
The FDA announced in August 2006 that it will form an internal FDA Nanotechnology Task Force. The group responsibilities will be to establish regulatory approaches in the development of safe and effective FDA-regulated products that use nanotechnology, affecting virtually every product category that FDA regulates—from pharmaceuticals and devices to cosmetics and food supplements. In July 2007 the FDA released a report that recommends the agency to consider guidance and steps to address benefits and risks of drugs and medical devices using nanotechnology.
Eschenbach (MD), a Food and Drug commissioner stated that “Nanotechnology holds enormous potential for use in a vast array of products”. Guidance would clarify what information needs to be supplied to the FDA when developing nanotechnology-based products and, as the uncertain nature of nanotechnology and the potential rapid development clearly highlights the need for predictable, transparent and consistent regulatory pathways. A need to identify and access data needs in the FDA regulatory process of nanotechnology and products and will cover both interactions and possible biological effects of nano-sized materials. The report also flags for a commitment to a more transparent public process when FDA develops its regulatory policies around nanotechnology. Public participation will ultimately win public confidence and be paramount when developing good policies in the agency’s oversight of nanotechnology and nanotech products.
Many of the products that incorporate nanotechnology are non-pharmaceuticals comprising materials and line extensions of these. In the next five years it is foreseen that we will see products in the field of solar energy, batteries, displays and e-paper, nanotubes and nanoparticle composites, catalysts, coatings, paints, alloys, insulation, filters, glues, abrasives, lubricants etc. The time to market these products varies and depends both on the technological complexity as well as the regulatory complexity and product life cycle as can be seen in Figure 1.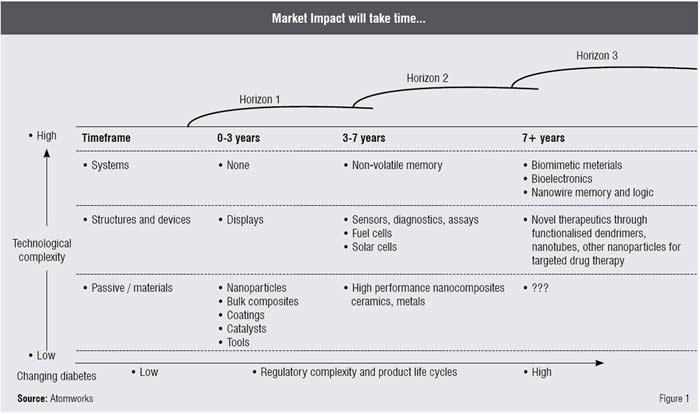
The sale of these products has increased substantially over the years; in 2004, the revenues were almost US$ 13 billion and are projected to be over US$ 500 billion by 2010. This figure is comparable to the total revenues of the pharmaceutical industry in 2004, surpassing US$ 500 billion this year. The driver for growth is surprisingly not NCEs or NBEs within the pharmaceutical sector, as the R&D spendings and cost increases the development time increases, thereby decreasing the time of exclusivity. Pharmaceutical companies claim that the non-invasive routes of administration for protein therapeutics are selected mainly to bring about differentiation, user compliance and Life Cycle Management (LCM). New dosage forms and reformulation pose a challenge to LCM of drugs due to various factors such as solubility, stability, potency and compliance / convenience. Poor solubility and frequent dosing schedules are two drivers of innovation in drug delivery for nanotechnology where the technology can be used to improve or control release / uptake of drug compounds.
We have already seen efforts in the area: NanoCrystals® from Elan Corporation (Rapamune®, Emand®, TriCor®, Megace®), Baxter DissoCubes®, SkyPharma NanoEdge® Abraxis Abraxane® and Bioaqueous Solution technology from Dow Pharma (license from University of Texas). Current trends in protein formulation and drug delivery lie in the selection of the route of administration of the NBE and drug delivery formulation, together with tailored device development. Typical examples are transdermal implants and sustained release and inhalation therapeutics; however, the latter has declined substantially at the moment due to the discontinuation of inhaled insulin therapy. Looking closer at the pipeline for protein and peptide drug delivery, the development in the inhaled field is similar to that of oral and injectables (SR) during Phase I and II and the industry is showing a substantial interest in this field.
From the above it can be concluded that future development of inhaled therapeutics will focus more on LCM of existing products along with development of NCEs / NBEs where improved particle design and engineering will be key development trends. The size, shape and composition are the main factors of interest to exploit further. Both the size and the shape factors such as structure, form and topology will have an influence on the drug delivery from the device as the binding or connecting forces can be moderated to improve small or nanoparticle dispersion during delivery.
Classically, drug delivery via inhalation has predominately centred on micron particles as these have been able to deliver defined metered dose during one or more inhalations. Switching to submicron or nanoparticles, the number of particles easily increases by a factor of thousand or million for the same dosing regime and the adhesive / cohesive forces multiply to the same extent. Exploiting options around shape and composition is a must to minimise the effect of cohesion / adhesion in drug delivery. The composition, shape, density and the total number of particles (e.g. the total surface coating of the lung) may have an effect on the dissolution rate, uptake and biodegradability of the particles when they reach the lung as compared to larger micron sized particles.
This also reflects the possibilities of using nanoparticles and nanomaterials in potential rapid topical drug delivery. Nanoparticles can also be a catalyst in the development of new carrier molecules and materials such as nanoshells and assembled structures. All of these features can of course be incorporated into the future inhalation therapy.
The inhalers that are offered today are divided into three segments—Dry Powder Inhalers (DPI), pressurised Metered Dose Inhalers (pMDI) and nebulisers or Soft Mist Inhalers (SMI). In DPI, the drug is formulated as a dry powder, containing the active drug component and excipient or carriers. A variety of DPIs are available today and they can have either a reservoir of powder or be supplied with a number of unit blisters / capsules for individual dosing. The pMDI and the SMI (nebuliser) use a dry powder formulation but the powder is suspended in a Hydro Fluoro Alkanes (HFA) or Chloro Fluoro Carbons (CFC) propellant in the case of pMDIs and in water in the case of a nebuliser or SMI. A pMDI is a reservoir system and often holds 60 to 120 doses in the reservoir. The SMI is a reservoir system containing multiple highly concentrated µl doses (typically10 to 50 µl) whereas the nebuliser uses a lower concentrated ml dose (typically 1 to 3 ml). Stability and degradation is an issue and is more accentuated in the water-based nebuliser / SMI systems than in pMDI and DPI systems due to the better chemical stability in non-aqueous systems.
Inhaler devices can of course be used for delivering nanoparticles, both as a drug and as a carrier system for various purposes. Care has to be taken as the implementation of nanoparticles in inhalation therapy poses some major challenges to the developer. Due to the nature of nanoparticles, they will give rise to many formulation issues when blended with excipients as the surface to area ratio is large compared to micron-sized particles and thus will have an impact on blending and aggregation properties of the formulation. For liquid (aqueous and HFA systems) aggregation resulting in flocculation and caking together with risk of adhesion to walls and device mechanism may have an impact on device dosing properties (uniformity and over time).
Dispersion of the particles can also pose some challenges, especially for DPIs where the patient inhalation effort drives the de-aggregation of particles, a factor that is known to vary substantially depending on the disease and state of the patient. The residue in the blister / capsule and device will also be dependent on the nature of the formulatin used and opens for both issues and possibilities when employing nanotechnology (particles and structures) in inhalation-based delivery of drugs. After solving these issues, there may still be other issues to address when looking at PK / PD profiles. This is especially critical of generic applications where equivalent PK / PD profiles and dosing to marketed products are essential. The difference in particle size and surface area coverage along with possible differences in dissolution and biodegradability / availability can have a major impact and must be addressed early in the development.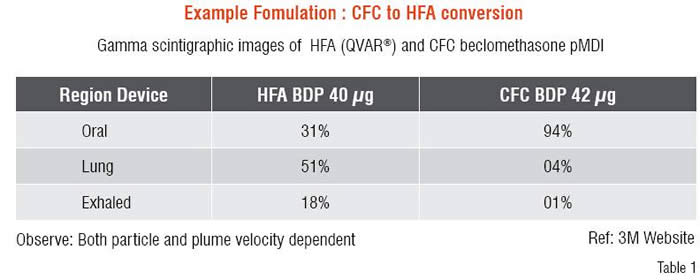
A wide range of possibilities in which nanoparticles can be used in inhalation therapy is felt since long. When IVAX and 3M developed the QVAR® pMDI system as a CFC to HFA conversion of an existing CFC product containing a micron-sized suspension, the resulting HFA formulation became a solution and not a suspension. When activating the QVAR® pMDI, the HFA boils off resulting in solid particles whose size depended on the drug concentration in the HFA formulation and the initial droplet size of the HFA droplet distribution. The resulting solid particles were much smaller than the suspended particles in the CFC suspension formulation and were more likely to penetrate into the lung as compared to the CFC suspension particles. The reported results can be seen in Table 1 which clearly shows an enhanced lung deposition of the QVAR® as compared to the conventional CFC product on the market. This clearly highlights opportunities for nanoparticle applications. But, it also highlights the differences in dosing and possible PK / PD issues for generica or line extension (LCM) applications.
The success of nanoparticle applications in the inhaled field depends on the feasibility of designing nanoparticle formulations that are stable and can be delivered successfully and repeatedly to the patient. Many issues need to be considered and thoroughly tested in order to achieve the goals set in the development phase. If this is successfully done, new options securing IP-enabling novel NCE / NBE or even line extension applications may be thought of. At the moment, this area is fairly unexploited generating new IP in the field giving a cutting edge to novel products in the future for those pharmaceutical companies working in the inhaled product segment.