Plant viruses are nano-sized particles with the natural capacity to transfer and release nucleic acids into eukaryotic cells. Plant virus-based nanoparticles (PVNPs) refer to plant viruses or virus-like particles (VLPs) and spherical nanoparticles (SNPs) which originated from plant viruses and have medical applications. With rapid developments in viral nanotechnology research, PVNPs are emerging as a beneficial nano-toolbox for cancer treatment through their ability to carry anticancer components and activate an anti-tumour immune system in vivo. Herein we briefly review the application of PVNPs as vehicles of therapeutic agents, immunotherapeutic agents, and as direct immunomodulators.
Nowadays, nanoparticles are a popular toolbox used in cancer research for improving the pharmaceutical capacity of therapeutic agents. Viral nanoparticles (VNPs) are naturally-occurring NPs that have emerged as key players. VNPs are structures between 30 -200 nm in diameter, having unique size- and shape-dependent physicochemical and biological properties. Depending on their origin, VNPs can belong to mammalian viruses, bacteriophages, or plant viruses. While VNPs exist with differences in shape, structure, and molecular components, they can share similar applications in cancer therapy. VNPs offer powerful platforms for vaccines, immunotherapy, and delivery of theranostic payloads. Due to cytotoxicity, unwanted immunologic reactions and adverse side effects, mammalian viruses influence VNP technology’s cost, complexity, and safety. With regard to these limitations, plant viruses or plant viral nanoparticles (PVNPs) have emerged as an alternative platform. The many properties of PVNPs make them good candidates for developing tumour therapy. These features include their lack of pathogenicity, biocompatibility and biodegradability properties in mammalian systems, stability in rigid environment conditions, simple external functionalisation by either single or multiple functional group expression, payload loading capacity and their inherent immunostimulatory effect. PVNPs include Brome mosaic virus (BMV), Red clover necrotic mosaic virus (RCNMV), Cowpea chlorotic mottle virus (CCMV), Cowpea mosaic virus (CPMV) Potato virus X (PVX), Tobacco mosaic virus (TMV), Alfalfa mosaic virus (AMV) that have been used as scaffolds in cancer research. Here, we present the functions and applications of PVNPs in cancer treatment.
PVNPs are potent platforms from the nano-toolbox for the treatment of cancer. Similar to synthetic NPs, the nature, structure, and physicochemical features of PVNPs determine their medical application. PVNPs are self-assembled protein coat (CP) units that form a hollow structure for entrapping nucleic acids. In the presence of nucleic acid, CPs self-assemble in icosahedral, filamentous, rod-like, and bacillus morphologies. In contrast, in the deprivation of nucleic acids, CPs self-assemble as hollow virus-like particles (VLP), or spherical nanoparticles (SNP). PVNPs used to combat cancer can manifest as two strategies: 1) as protein nanovehicles for loading anticancer therapeutic agents to increase their therapeutic efficacy, and 2) as immunomodulatory agents for enhancing anti-tumour immune responses.
The unique structural and chemical properties of PVNPs make them ideal nanocarriers. PVNPs’ empty internal cavities, surface groups, and open/closed conformations enable cargo to be loaded. Therapeutic cargos are usually loaded in PVNPs via noncovalent (i.e., self-assembly, infusion, and charge/ ionic interaction) and covalent (i.e., genetically and chemically) mechanisms (reviewed by refs). Genetic manipulation is often used for displaying target ligands, antigenic structures, or specific amino acids or small peptides as tags (e.g. SNAP-tag) for further functionalisation. The self-assembly process is based on the caging of coat protein around a cargo. PVNP chemical modifications can be achieved through conjugation reactions (bioconjugate chemistry, click chemistry). Some PVNPs can trap cargo via a pore-opening mechanism in response to environmental conditions (i.e., pH and salt concentrations). PVNPs possess a negative/positive charge within a biological pH, and thus, they load charged cargos via electrostatic interactions.
More importantly and for addressing human solid tumours, PVNPs can target and transfer their cargo into the tumour microenvironment (TME) and within tumour cells themselves. Accumulation of PVNPs in TME is determined by size and blood distribution. PVNPs tend to accumulate in TME much more than in normal tissue, because of leaky vasculature and poor lymphatic drainage, as well as enhanced permeability and retention (EPR) effect. For targeting tumour cells, PVNPs don't require specific ligands by nature, however, they could be manipulated or engineered to display agonist ligands of tumour cell-membrane receptors, in order to directly deliver and transfer their cargo. Generally, structural properties (e.g., size, charge, and shape), various methods of cargo loading, and bioengineering all provide PVNPs for non-targeted and targeted delivery of therapeutic agents (e.g., small molecule drugs, nucleic acids, peptides, and proteins) and immunotherapeutic agents for cancer treatment (Figure 1).
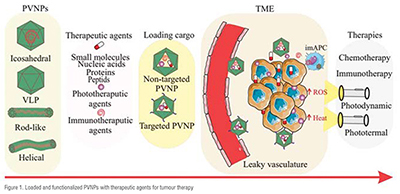
Therapeutic agents consisting of chemical and biological drugs can target and kill tumour cells via specific mechanisms. Traditional therapeutic drugs are highly effective to kill cancerous cells. However, their systemic administration have certain disadvantages such as lower bioavailability, minimal effectiveness, and severe side effects. Therefore, PVNP-based formulations have been designed as nano vehicles toward improving the pharmacological profiles of therapeutic drugs (Table 1). As mentioned above, PVNPs can load therapeutic agents via the exterior and/or interior of the capsid surface using covalent or non-covalent interactions. The nanoparticulate features (size, shape, charge, and surface functionalities) of PVNPs, and leaky nature of the vasculature (or EPR effect) of tumours lead to the accumulation of loaded PVNPs with therapeutic agents in the TME.
For example, charge-driven drug loading strategies were applied for encapsulating mitoxantrone (MTO) into TMV, a 300 × 18 nm nanorod containing a 4 nm-wide channel, and lined with glutamic acids. The negative charge of the glutamic acids allows for electrostatic interactions with the positively charged MTO, thus allowing for pH dependent drug-loading and release. In vitro and in vivo results confirmed that MTO maintained its efficacy when delivered by TMV in a panel of cancer cell lines in addition to a triple negative breast cancer mouse model. Another drug for loading into the nano-channel of TMV has been the active dictation form of cisplatin (cisPt2+), making use of the negatively charged Glu acid side chains that line the interior channel of TMV. TMV-cisPt exhibited superior efficacy vs free cisPt in ovarian tumour mouse models.
The greatest challenge in PVNP -based therapeutic agents is the low efficiency of delivery to tumour cells. PVNP-based targeted delivery is designed using an overexpression of tumour cell biomarkers -based agonist ligands for targeting, binding and delivering the payload to tumour cells. For example, display of (((S)-5-amino-1-carboxypentyl) carbamoyl)-L-glutamic acid (DUPA), a specific ligand to prostate-specific membrane antigen (PSMA), in TMV and loaded with MTO increased cytotoxicity of PSMA+ prostate cancer threefold, and is a promising therapeutic strategy. PVNPs displaying folic acid, GE11 (a small peptide with 12 amino acids), HER2 ligands (trastuzumab, CH401 epitope), tumour-homing peptides (THPs, IR780 iodide, F3), TRAIL (tumour-necrosis factor related apoptosis-inducing ligand), arginine–glycine–aspartate (RGD) peptide, and Asp-Gly-Glu-Ala (DGEA) peptide, have been shown to selectively target tumour cells.
Photodynamic therapy (PDT) and photothermal therapy (PTT) are promising avenues for improving the efficacy of cancer treatment. PVNPs can deliver photo activated therapeutic agents for inducing cytotoxicity via PDT and PTT. In this process, PD-PVNP accumulates in TME, and then is activated by light to generate reactive oxygen species (ROS) such as hydrogen peroxide, hydroxyl radicals, superoxide anions or singlet oxygen, which consequently cause cytotoxicity to tumour cells. For example, the cationic photosensitiser Porphyrin encapsulated the interior channel of TMV via electrostatic interactions to improve cell uptake and efficacy compared to free photosensitisers in a melanoma model. In another study, the incorporation of Zn-EpPor PS through electrostatic interactions with the carboxyl dendron attached to CPMV improved 2-fold the uptake efficacy and cell death in a B16F10 melanoma cell line compared to free PS. Free Zn-Por3+ displayed the greatest cell toxicity and the loading of Zn-Por into TMV and TMGMV resulted in slightly decreased cell toxicity in vitro. In contrast, targeted Zn-Por -TMV (with the nucleolin-specific F3 peptide accumulating at the cancer cell surface) can increase tumour cytotoxicity fivefold.
Photothermal therapy (PT) includes a PVNP associated with a photothermal agent. In this process, PT-PVNP accumulates in TME, and becomes activated by light to generate heat. Gd-TMV– polydopamine (PDA), with a strong near-infrared absorption and a high photothermal conversion efficiency (28.9%), offer promising results for effectively killing PC-3 prostate cancer cells in vivo and in cancer models. Coating of TMV with polydopamine (PDA), as a PTT agent was demonstrated to increase anti-tumour efficacy based on PTT- immunotherapy in B16F10 dermal melanoma in C57BL/6 mice. Targeted Cowpea chlorotic mottle virus (CCMV) capsids with tumour homing peptide F3 (via genetic engineering), and loaded with near-infrared fluorescent dye IR780 iodide, (F3-CCMV-IR780 NPs), displayed excellent molecular targeting PTT to nucleolin receptor over-expressed on the surface of MCF-7 tumour cells.
Nanocarrier properties of PVNPs have prepared them for the delivery of immunotherapeutic agents to the target site (e.g. TME, antigen presenting cells (APCs), and other components of the immune system) to improve the therapeutic index. Toward this goal, encapsulation of oligodeoxynucleotides (CpG ODNs, ODN1826), as agonists of Toll-like receptor 9 (TLR 9) into CCMV and TLR 7 agonist (1V209) into TMV has been shown to slow tumour growth and prolong survival in mouse models of colon cancer and melanoma. This demonstrated that bioconjugation with the anti-PD-1 peptide SNTSESF (AUNP) into CPMV (the CPMV-AUNP formulation) increased the anti-tumour efficacy of AUNP into ovarian cancer cells compared to free peptide.
PVNPs structural properties (i.e., size, shape and rigidity) can transfer and stimulate APCs within the lymph node. For example, to overcome immunological tolerance against HER2-positive tumour cells, integrated HER2 epitopes loaded onto Potato virus X (PVX), and CPMV acted as vaccines without requiring additional adjuvants to induce a strong and sustained anti-HER2 immune response. Similarly, a VLP-based vaccine has been designed via click chemistry with the attachment of the HER2-derived CH401 peptide epitope into PhMV. Results have shown that PhMV-based vaccine enhanced anticancer immunity by high titers of HER2-specific immunoglobulins, increased the toxicity of antisera to DDHER2 tumour cells, and prolonged survival of the vaccinated vs. naïve BALB/C mice. Testis antigen NY-ESO-1 is an attractive antigenic target for cancer vaccines. Displaying multiple copies of human HLA-A2 restricted peptide antigen NY-ESO-1157–165 into CPMV enhances uptake and activation of APCs and stimulates a potent CD8+ T cell response. This study shows the potential of CPMV-NY-ESO-1 vaccine against NY-ESO-1+ malignancies. These studies also explain how PVNP formulations are expected to exhibit prolonged tumour residence and favorable intratumoural distribution. (Table 1)
The inherent immunogenicity of PVNPs have provided them with tremendous potential as direct immunomodulators to activate the innate immune response. The function of PVNPs in immunomodulation depends on their structural components, capsid protein and genome. They can act as non-self (foreign), or danger signals, and active pattern recognition receptors (PRRs) on immune cells carrying Toll like receptors (TLRs). For example CPMV (virion) and empty CPMV (eCPMV, without the nucleic acid) capsids are recognised by MyD88-dependent TLR2 and TLR4, and the release of the ssRNA contained within CPMV and PapMV is recognized by TLR7. It was demonstrated that PVNPs can act as pathogen-associated molecular patterns (PAMPs) for TLR of the surface (1, 2, 4, 5, and 6) or the endosome (TLRs 3, 7, 8, and 9) on APCs.
In situ vaccination (ISV) uses intratumoural injection of PVNPs to activate the innate and adaptive immune system in TME. Virions with active or deactivated nucleic acids and VLP can be used for ISV. PVNPs-ISV therapy leads to changes in cytokine levels within the TME, and reprogram and repolarise suppressed innate immune cells toward an anti-tumour phenotype. They also induce the recruitment of innate immune cells that are cytotoxic to cancer cells. PVNPs can induce pro-inflammatory cytokine production such as interleukin (IL)-1, IL-6 and IL-12, interferon (IFN)-, and IFN- that potentiate to induce an adaptive immune response (Figure 2). A typical PVNP used for ISV is CPMV. The CPMV capsid triggers TLRs 2, 4 and the ssRNA within CPMV activates TLR 7, and receptor signalling cascades lead to the release of immunostimulatory cytokines such as IL-1, IL-12, IFN-, chemokine ligand 3, macrophage inflammatory protein-2, and granulocyte-macrophage colony-stimulating factor (GM-CSF).
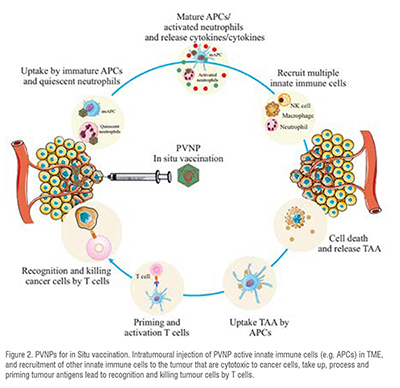
The tumour acts as a resource of antigens in ISV, and upon tumour antigen release in the TME, processing and priming by the APCs leads to the activation of the induction of systemic and tumour-specific immune responses. To date, the in situ injection of CPMV, Cowpea severe mosaic virus (CPSMV) and Tobacco ring spot virus (TRSV), Cowpea Chlorotic mottle virus (CCMV), Physalis mosaic virus (PhMV), and Sesbania mosaic virus (SeMV), TMV, PVX, Papaya mosaic virus (PapMV), Alfalfa mosaic virus (AMV) have demonstrated anti-tumour potentials in mouse models.
PVNP-mediated ISV is generally effective only against small tumours, and most patients do not respond to PVNP monotherapy. Combining multiple treatment regimens with PVNP -based ISV could form the basis for success. For example, immune checkpoint therapy (ICT) has the potential to treat cancer by removing the immunosuppressive brakes on T cell activity. It is shown that combined treatment with CPMV and selected checkpoint-targeting antibodies, specifically anti-PD-1 antibodies, or agonistic OX40-specific antibodies, reduced tumour burden, prolonged survival, and induced tumour antigen-specific immunologic memory to prevent relapse in mouse tumour models. In addition, CPMV-based in situ vaccination combined with systemic low-dose CPA chemotherapy achieved impressive synergistic efficacy against 4T1 tumours. Low doses of CPA induce pro-immunogenic activity in tumour cells, including the hallmarks of immunogenic cell death (ICD). Activated APCs with CPMV ISV induce IL-12, IFN-, and IFN-and potentiated to induce an adaptive immune response. Data indicated that the combination of RT + CPMV enhanced efficacy over RT alone, and that this may be attributed to an expansion of T cells within the tumours. Many investigations examined the combination of PVNP in situ vaccination with chemotherapy, radiation therapy, checkpoint immunotherapies. Overall, multifunctional PVNPs can combine multiple treatment modalities into a single platform with ISV.
The development of plant viruses as expression vectors for pharmaceutical production has played an integral role in the emergence of plants as inexpensive and facile systems for the generation of therapeutic proteins. More recently, plant viruses have been designed as non-toxic nanoparticles which can target a variety of cancers via loading conventional therapeutic agents, tumour antigens, and immunotherapeutic agents. The tendency of PVNPs to interact with and become phagocytosed by innate immune cells can empower the immune system to slow or even reverse tumour progression.
References
1. Wen, A.M. and N.F. Steinmetz, Design of virus-based nanomaterials for medicine, biotechnology, and energy. Chemical Society Reviews, 2016. 45(15): p. 4074-4126.
2. Beatty, P.H. and J.D. Lewis, Cowpea mosaic virus nanoparticles for cancer imaging and therapy. Advanced Drug Delivery Reviews, 2019. 145: p. 130-144.
3. Yildiz, I., S. Shukla, and N.F. Steinmetz, Applications of viral nanoparticles in medicine. Current opinion in biotechnology, 2011. 22(6): p. 901-908.
4. Azizi, M., et al., Multifunctional plant virus nanoparticles: An emerging strategy for therapy of cancer. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 2022: p. e1872.
5. Nkanga, C.I. and N.F. Steinmetz, The pharmacology of plant virus nanoparticles. Virology, 2021. 556: p. 39-61.
6. Shahgolzari, M., et al., Plant viral nanoparticles for packaging and in vivo delivery of bioactive cargos. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 2020. 12(5): p. e1629.
7. Bruckman, M.A., A. VanMeter, and N.F. Steinmetz, Nanomanufacturing of Tobacco Mosaic Virus-Based Spherical Biomaterials Using a Continuous Flow Method. ACS Biomaterials Science & Engineering, 2015. 1(1): p. 13-18.
8. Czapar, A.E. and N.F. Steinmetz, Plant viruses and bacteriophages for drug delivery in medicine and biotechnology. Current opinion in chemical biology, 2017. 38: p. 108-116.
9. Glidden, M.D., et al., Application of engineered viral nanoparticles in materials and medicine. Chemoselective and Bioorthogonal Ligation Reactions: Concepts and Applications, 2017. 2: p. 631-710.
10. Shahgolzari, M., H. Dianat-Moghadam, and S. Fiering, Multifunctional plant virus nanoparticles in the next generation of cancer immunotherapies. Seminars in Cancer Biology, 2022. 86: p. 1076-1085.
11. Colombo, M., et al., Protein Oriented Ligation on Nanoparticles Exploiting O6‐Alkylguanine‐DNA Transferase (SNAP) Genetically Encoded Fusion. Small, 2012. 8(10): p. 1492-1497.
12. Yildiz, I., et al., Infusion of imaging and therapeutic molecules into the plant virus-based carrier cowpea mosaic virus: cargo-loading and delivery. Journal of controlled release, 2013. 172(2): p. 568-578.
13. Lockney, D.M., et al., The Red clover necrotic mosaic virus capsid as a multifunctional cell targeting plant viral nanoparticle. Bioconjugate chemistry, 2011. 22(1): p. 67-73.
14. Lin, R.D. and N.F. Steinmetz, Tobacco mosaic virus delivery of mitoxantrone for cancer therapy. Nanoscale, 2018. 10(34): p. 16307-16313.
15. Venkataraman, S., et al., Plant virus nanoparticles for anti-cancer therapy. Frontiers in Bioengineering and Biotechnology, 2021: p. 817.
16. Kharkar, P.B., et al., Nanosystems for oral delivery of immunomodulators, in Nanostructures for Oral Medicine. 2017, Elsevier. p. 295-334.
17. Shahgolzari, M., et al., Multifunctional Plant Virus Nanoparticles for Targeting Breast Cancer Tumors. Vaccines, 2022. 10(9): p. 1431.
18. Zhao, Z., A. Simms, and N.F. Steinmetz, Cisplatin-Loaded Tobacco Mosaic Virus for Ovarian Cancer Treatment. Biomacromolecules, 2022. 23(10): p. 4379-4387.
19. Shukla, S., et al., Tobacco mosaic virus for the targeted delivery of drugs to cells expressing prostate-specific membrane antigen. RSC advances, 2021. 11(33): p. 20101-20108.
20. Ren, Y., S.M. Wong, and L.-Y. Lim, Folic acid-conjugated protein cages of a plant virus: a novel delivery platform for doxorubicin. Bioconjugate chemistry, 2007. 18(3): p. 836-843.
21. Chariou, P.L., et al., Detection and imaging of aggressive cancer cells using an epidermal growth factor receptor (EGFR)-targeted filamentous plant virus-based nanoparticle. Bioconjugate chemistry, 2015. 26(2): p. 262-269.
22. Gupta, S., et al. Plant virus-resembling optical nano-materials conjugated with anti-EGFR for targeted cancer imaging. in Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications IV. 2012. SPIE.
23. Shukla, S., et al., Presentation of HER2 epitopes using a filamentous plant virus-based vaccination platform. Journal of Materials Chemistry B, 2014. 2(37): p. 6249-6258.
24. Esfandiari, N., M.K. Arzanani, and M. Koohi-Habibi, The study of toxicity and pathogenicity risk of Potato Virus X/Herceptin nanoparticles as agents for cancer therapy. Cancer Nanotechnology, 2018. 9: p. 1-13.
25. Le, D.H., U. Commandeur, and N.F. Steinmetz, Presentation and delivery of tumor necrosis factor-related apoptosis-inducing ligand via elongated plant viral nanoparticle enhances antitumor efficacy. ACS nano, 2019. 13(2): p. 2501-2510.
26. Hovlid, M.L., et al., Guiding plant virus particles to integrin-displaying cells. Nanoscale, 2012. 4(12): p. 3698-3705.
27. Hu, H., et al., Physalis mottle virus-like nanoparticles for targeted cancer imaging. ACS applied materials & interfaces, 2019. 11(20): p. 18213-18223.
28. Steinmetz, N.F., Plant virus particles for delivery of photosensitive agents. 2020, Google Patents.
29. Lee, K.L., et al., High aspect ratio nanotubes formed by tobacco mosaic virus for delivery of photodynamic agents targeting melanoma. ACS biomaterials science & engineering, 2016. 2(5): p. 838-844.
30. Wen, A.M., et al., Utilizing viral nanoparticle/dendron hybrid conjugates in photodynamic therapy for dual delivery to macrophages and cancer cells. Bioconjugate chemistry, 2016. 27(5): p. 1227-1235.
31. Chariou, P.L., et al., Let there be light: Targeted photodynamic therapy using high aspect ratio plant viral nanoparticles. Macromolecular Bioscience, 2019. 19(5): p. 1800407.
32. Hu, H., et al., Polydopamine-decorated tobacco mosaic virus for photoacoustic/magnetic resonance bimodal imaging and photothermal cancer therapy. Nanoscale, 2019. 11(19): p. 9760-9768.
33. Nkanga, C.I., O.A. Ortega-Rivera, and N.F. Steinmetz, Photothermal immunotherapy of melanoma using TLR-7 agonist laden tobacco mosaic virus with polydopamine coat. Nanomedicine: Nanotechnology, Biology and Medicine, 2022. 44: p. 102573.
34. Wu, Y., et al., Targeted cowpea chlorotic mottle virus-based nanoparticles with tumor-homing peptide F3 for photothermal therapy. Biotechnology and Bioprocess Engineering, 2017. 22: p. 700-708.
35. Cai, H., S. Shukla, and N.F. Steinmetz, The antitumor efficacy of CpG oligonucleotides is improved by encapsulation in plant virus‐like particles. Advanced functional materials, 2020. 30(15): p. 1908743.
36. Gautam, A., et al., Plant viral nanoparticle conjugated with anti-PD-1 peptide for ovarian cancer immunotherapy. International Journal of Molecular Sciences, 2021. 22(18): p. 9733.
37. Bachmann, M.F. and G.T. Jennings, Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nature Reviews Immunology, 2010. 10(11): p. 787-796.
38. Shukla, S., et al., Plant viral nanoparticles-based HER2 vaccine: Immune response influenced by differential transport, localization and cellular interactions of particulate carriers. Biomaterials, 2017. 121: p. 15-27.
39. Shukla, S., et al., A viral nanoparticle cancer vaccine delays tumor progression and prolongs survival in a HER2+ tumor mouse model. Advanced therapeutics, 2019. 2(4): p. 1800139.
40. Hu, H. and N.F. Steinmetz, Development of a virus-like particle-based anti-HER2 breast cancer vaccine. Cancers, 2021. 13(12): p. 2909.
41. Dianat-Moghadam, H., et al., Engaging stemness improves cancer immunotherapy. Cancer Letters, 2022: p. 216007.
42. Patel, B.K., et al., Cowpea Mosaic Virus (CPMV)-Based Cancer Testis Antigen NY-ESO-1 Vaccine Elicits an Antigen-Specific Cytotoxic T Cell Response. ACS Applied Bio Materials, 2020. 3(7): p. 4179-4187.
43. Aljabali, A.A., et al., Cpmv-dox delivers. Molecular pharmaceutics, 2013. 10(1): p. 3-10.
44. Zeng, Q., et al., Cucumber mosaic virus as drug delivery vehicle for doxorubicin. Biomaterials, 2013. 34(19): p. 4632-4642.
45. Alemzadeh, E., et al., Enhanced anti-tumor efficacy and reduced cardiotoxicity of doxorubicin delivered in a novel plant virus nanoparticle. Colloids and Surfaces B: Biointerfaces, 2019. 174: p. 80-86.
46. Arcangeli, C., et al., Structure-based design and experimental engineering of a plant virus nanoparticle for the presentation of immunogenic epitopes and as a drug carrier. Journal of Biomolecular Structure and Dynamics, 2014. 32(4): p. 630-647.
47. Lico, C., et al., Tomato Bushy Stunt Virus Nanoparticles as a Platform for Drug Delivery to Shh-Dependent Medulloblastoma. International Journal of Molecular Sciences, 2021. 22(19): p. 10523.
48. Madden, A.J., et al., Pharmacokinetics and efficacy of doxorubicin-loaded plant virus nanoparticles in preclinical models of cancer. Nanomedicine, 2017. 12(20): p. 2519-2532.
49. Bruckman, M.A., et al., Tobacco mosaic virus-based protein nanoparticles and nanorods for chemotherapy delivery targeting breast cancer. Journal of Controlled Release, 2016. 231: p. 103-113.
50. Lam, P., R.D. Lin, and N.F. Steinmetz, Delivery of mitoxantrone using a plant virus-based nanoparticle for the treatment of glioblastomas. Journal of Materials Chemistry B, 2018. 6(37): p. 5888-5895.
51. Shukla, S., et al., Affinity of plant viral nanoparticle potato virus X (PVX) towards malignant B cells enables cancer drug delivery. Biomaterials science, 2020. 8(14): p. 3935-3943.
52. Kernan, D.L., et al., Featured article: Delivery of chemotherapeutic vcMMAE using tobacco mosaic virus nanoparticles. Experimental Biology and Medicine, 2017. 242(14): p. 1405-1411.
53. Masarapu, H., et al., Physalis mottle virus-like particles as nanocarriers for imaging reagents and drugs. Biomacromolecules, 2017. 18(12): p. 4141-4153.
54. Esfandiari, N., et al., A new application of plant virus nanoparticles as drug delivery in breast cancer. Tumor Biology, 2016. 37: p. 1229-1236.
55. Lebel, M.-È., et al., Potentiating cancer immunotherapy using papaya mosaic virus-derived nanoparticles. Nano letters, 2016. 16(3): p. 1826-1832.
56. Mao, C., et al., Cowpea mosaic virus stimulates antitumor immunity through recognition by multiple MYD88-dependent toll-like receptors. Biomaterials, 2021. 275: p. 120914.
57. Beiss, V., et al., Cowpea mosaic virus outperforms other members of the Secoviridae as in situ vaccine for cancer immunotherapy. Molecular pharmaceutics, 2022. 19(5): p. 1573-1585.
58. Jung, E., et al., Inactivated Cowpea Mosaic Virus for In Situ Vaccination: Differential Efficacy of Formalin vs UV-Inactivated Formulations. Molecular Pharmaceutics, 2022. 20(1): p. 500-507.
59. Koellhoffer, E.C., et al., Inactivated cowpea mosaic virus in combination with OX40 agonist primes potent antitumor immunity in a bilateral melanoma mouse model. Molecular pharmaceutics, 2022. 19(2): p. 592-601.
60. Chariou, P.L., et al., In situ vaccine application of inactivated CPMV nanoparticles for cancer immunotherapy. Materials advances, 2021. 2(5): p. 1644-1656.
61. Wang, C., S.N. Fiering, and N.F. Steinmetz, Cowpea mosaic virus promotes anti‐tumor activity and immune memory in a mouse ovarian tumor model. Advanced therapeutics, 2019. 2(5): p. 1900003.
62. Lizotte, P., et al., In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nature nanotechnology, 2016. 11(3): p. 295-303.
63. Cai, H., et al., Cowpea mosaic virus immunotherapy combined with cyclophosphamide reduces breast cancer tumor burden and inhibits lung metastasis. Advanced Science, 2019. 6(16): p. 1802281.
64. Murray, A.A., et al., In situ vaccination with cowpea vs tobacco mosaic virus against melanoma. Molecular pharmaceutics, 2018. 15(9): p. 3700-3716.
65. Chung, Y.H., B.A. Volckaert, and N.F. Steinmetz, Metastatic Colon Cancer Treatment Using S100A9-Targeted Cowpea Mosaic Virus Nanoparticles. Biomacromolecules, 2022. 23(12): p. 5127-5136.
66. Shahgolzari, M., et al., Alfalfa mosaic virus nanoparticles-based in situ vaccination induces antitumor immune responses in breast cancer model. Nanomedicine, 2020. 16(2): p. 97-107.
67. Hersey, P., Impediments to successful immunotherapy. Pharmacology & therapeutics, 1999. 81(2): p. 111-119.
68. Patel, R., et al., Radiation therapy combined with cowpea mosaic virus nanoparticle in situ vaccination initiates immune-mediated tumor regression. ACS omega, 2018. 3(4): p. 3702-3707.
69. Wang, C. and N.F. Steinmetz, A combination of cowpea mosaic virus and immune checkpoint therapy synergistically improves therapeutic efficacy in three tumor models. Advanced functional materials, 2020. 30(27): p. 2002299.
70. Lee, K.L., et al., Combination of plant virus nanoparticle-based in situ vaccination with chemotherapy potentiates antitumor response. Nano letters, 2017. 17(7): p. 4019-4028.
71. Wang, C. and N.F. Steinmetz, CD47 blockade and cowpea mosaic virus nanoparticle in situ vaccination triggers phagocytosis and tumor killing. Advanced healthcare materials, 2019. 8(8): p. 1801288.
72. Mao, C., et al., In situ vaccination with cowpea mosaic virus elicits systemic antitumor immunity and potentiates immune checkpoint blockade. Journal for ImmunoTherapy of Cancer, 2022. 10(12): p. e005834.