Growing adoption of precision medicine has led to more in-depth patient screening for inclusion in clinical trials. It has also increased the inclusion/exclusion criteria for Human Biological Specimens (HBS) for life sciences research. That makes it harder for drug and diagnostic development researchers to acquire appropriate HBS for their studies.
For many drug discovery programmes, HBS form the foundation of many hypotheses and contribute to research that is the basis for go/no-go decisions during the development process. But what if the HBS used in the research do not properly represent the clinical situation with the appropriate representative donors and diagnoses? The resulting research may not align with the clinical setting and unnecessary drug attrition may occur.
To fully understand how this may occur, it is important to know how HBS for research are obtained. To begin with, in most cases an Institutional Review Board (IRB) consented donor undergoes a doctor’s exam or surgical resection as part of the standard of care for their disease. At that point, an HBS is collected, generally either biofluid or tissue. Those HBS may be collected prospectively, with known donor consent and written authorisation to donate said specimen for research. Alternatively, samples may be collected retrospectively, in which case consent is not required prior to collection. In those cases, the HBS is not specifically collected for research but may be a sample destined to be discarded after appropriate tests are performed and the identity of the donor cannot readily be determined from coded private information or the specimen itself. It is important that these remnant samples are collected with the appropriate regulatory oversight.
In the best case scenario for blood-derived and non-blood derived biological fluids, HBS are prospectively collected according to a specific protocol with associated clinical data that define whether the patient meets specific inclusion and exclusion criteria. However, based on research needs and standard of care, this may not be feasible. Thus, remnant samples with the appropriate donor criteria, such as lab test results within specific ranges, may be procured instead. Any inconsistency or discrepancies in the clinical data must be flagged immediately for resolution with the specimen provider. If they cannot be resolved, the HBS should be quarantined and not used in further analysis.

For tissue samples, the best case scenario has HBS prospectively collected by a surgeon and surgical team when a patient undergoes surgical resection of the diseased tissue, together with surrounding non-diseased tissue to ensure the compromised tissue is completely removed. However, based on research needs and standard of care, this may not be feasible. Thus, upon IRB approval of waiver of consent, remnant samples with the appropriate donor criteria, such as clinical diagnosis, may be acquired from pathological archives instead. As with biological fluids, any inconsistency or discrepancies in the clinical data must be flagged immediately for resolution with the specimen provider. If they cannot be resolved, the HBS should be quarantined and not used in further analysis.
Tissue samples, whether collected prospectively or retrospectively, are unique and certain issues may arise that impact downstream use of the tissue blocks processed following a surgical procedure. For example, imagine a scenario where a patient is undergoing surgical resection of a lung adenocarcinoma. The surgeon and surgical team resect the tumour with surrounding non-neoplastic tissue to ensure the tumour is completely removed from the patient. That tumour mass is then collected and processed by speciallytrained personnel. Generally within 30 minutes of removal from the donor, the tissue is placed in formalin or flash frozen for fixation. Depending on the size of the tissue sample, it may be sectioned into smaller tissue fragments to allow proper fixation and microscopic examination.
Macroscopically, it can often be difficult to distinguish tumour from normal or diseased lung tissue; the tumour can be intermixed with haemorrhage, fibrosis, diseased tissue or necrosis.
In addition, it is known that tumours are heterogeneous, and thus the tumour that is excised may have a variation in diagnosis because of the greater volume of tumour that is available to examine microscopically compared to a small biopsy. For example, a lung adenocarcinoma could have areas of squamous cell carcinoma not present on the original needle core biopsy and should be classified as adenosquamous carcinoma.
If the average lung cancer tumour dimension is roughly three centimetres or 1.5 inches upon resection, this tumour mass would need to be cut into approximately three to five tissue blocks to allow proper fixation via either formalin or flash freezing for microscopic examination. This could result in three to five tissue blocks that contain a mix of tumour and unwanted non-neoplastic tissue that could merit a different diagnosis. This situation provokes several questions, as follows:
These questions can all be answered through an independent board-certified pathologist review of each tissue specimen used in the research process. Pathologist review of HBS provides quality control and assures that the correct tissue is analysed with the right anatomic site and diagnosis, ensuring the validity of research data and accelerating progress toward actionable data. Board-certified pathologists use their knowledge and understanding of current medical practices and standards to diagnosis the tissue block of interest accurately and verify that information matches the clinical data provided by the donor center.
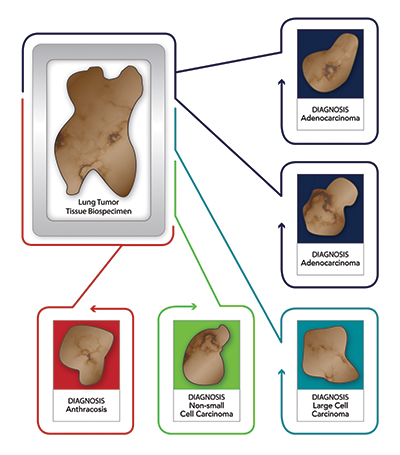
In the previous scenario with the three to five lung cancer tissue blocks, each block will undergo a separate review and receive its own diagnosis based on the tissue that is present in the representative H&E slide of that specific block. Thus, while the first two blocks may have a confirmed denocarcinoma
diagnosis, the third block may have a confirmed pneumonia diagnosis, the fourth block could show only fibrosis and inflammation, and the fifth block anthracosis (Figure 1).
In this specific case, the clinical diagnosis provided by the site was adenocarcinoma of the lung, but as can be seen in this scenario only two of the five blocks could be confirmed to be adenocarcinoma. Most importantly, using the blocks that showed only pneumonia or fibrosis versus any of the blocks with tumour obviously would have a significant negative impact on research results.
In addition, in the lung adenocarcinoma example, a pathologist could use immuno-stains to ensure that it does not represent a metastasis. Immunostaining could also refine or change the diagnosis. Presence or absence of a specific marker, such as TTF1, Napsin A, Chromogranin or Synaptophysin, is used to distinguish small cell lung cancer from non-small cell lung cancer as well as for identifying glandular or squamous differentiation and is thus important for an accurate diagnosis. Without this valued workup, the use of a specific tissue block may again negatively impact results.
Beyond confirming an accurate diagnosis, review of tissue blocks by a board-certified pathologist offers additional beneficial insights that ensure the validity of research outcomes and downstream decisions. These benefits include determining the percentage of different types of tissue within a block, tissue markup, quantitative measurements, grading slide quality or interpretation of specific research or prognostic immune-stains.
Reviewing these benefits individually will help researchers to understand where they add value. First, it is critical to understand the percentage of different types of tissue within each block used in a research experiment. As discussed above, a single block of tumour tissue may contain other unwanted tissue. For tumour-specific research, it is important to understand what percent of any specific block of tissue is tumour, with generally acceptable levels being 30-90 per cent. Knowing the percentage of diseased tissue is important in many other therapeutic research areas as well. Confirming that there is a minimal level of necrosis, generally less than 10 per cent, is useful in many downstream assays as necrotic tissue and signals can interfere with sensitivity assays. Depending on research needs, it is also important to know if there is an immune cell presence within a tissue block. Having each block reviewed by a board-certified pathologist, who provides these estimations, is essential to achieving actionable data. Further information can provide insight into other differences between specimens that may be important in downstream analysis.
Some diseases have validated prognostic stains that are required to determine specific treatment options. One of the most important of these is used for breast cancer. The expression pattern for these three receptors – Estrogen (ER), Progesterone (PR), and Human Epidermal growth factor Receptor 2 (HER2) – is essential in determining the clinical treatment of all breast cancer patients and thus is essential to many researchers studying this area.
As these are validated clinical assays, and their expression is known to be heterogeneous across a single breast cancer specimen, it is important that these levels are determined on a blockspecific basis. It is equally important that a board-certified pathologist determines the staining intensity and calls whether a specimen is positive or negative in expression for each of these markers consistent with the validated clinical parameters. In a similar fashion, pathologists can also be used to interpret research stains because they have a thorough understanding of the requirements needed to validate any stain.
At times, it is important to have the specific areas of interest, such as tumour, disease, necrosis or other, marked on the slides themselves. A pathologist can markup slides cut and created from tissue blocks to allow macro dissection or other determinations by automated or manual systems, thus ensuring proper input into molecular assays. Pathologists can also provide quantitative measurement of pathological lesions or other areas of significance for density, frequency, coverage, etc. Correlation of these quantitative measurements with clinical data can further validate that the proper tissue blocks are being used and that the research results are in line with the diagnosis of the donor cohort. An independent assessment of these factors can be important in accelerating research and achieving actionable data.
Conclusion
The application of precision medicine is growing across multiple therapeutic areas, most obviously in oncology. Ensuring that research aligns with clinical goals is essential to successful drug and companion diagnostic development. As reviewed here, there are inherent weaknesses in the HBS that are used in these research settings. Having a board-certified pathologist review tissue specimens truly impacts research and successful outcomes. Starting with the right HBS from the right donor with the right diagnosis will set research on the correct path. Marrying the right sample with the right clinical data, associated social-demographic information, mutation or genotype data, and potential outcome data under proper regulatory and quality review is a complex process. Therefore, it is critical that researchers work with HBS suppliers that have in-depth knowledge of regulatory requirements and expertise to deliver high-quality HBS.