Antibiotic resistance is a major threat posed by pathogenic bacteria to human health globally. It occurs through the destructive catalytic hydrolysis of -lactam antibiotics by metallo- -lactamases (MBLs) produced by the organisms. Some small-molecules are herein presented as potent broad-spectrum inhibitors of clinically relevant MBLs with ideal safety profiles for further studies.
Incessant resistance of pathogenic bacteria to almost all forms of antibiotics continues to pose a major threat to human well-being worldwide. Consequently, antibiotic resistance (AR) has become renowned and constitutes an unimaginable burden on the global healthcare and economic systems. For instance, an international collaborative analysis on the global burden of AR by Murray and co-workers provided an alarming rate of occurrence, epidemiology, morbidity and mortality associated with AR across the human populations in their pedigrees. Accordingly, in 2019, about 4.95 million deaths were estimated to have resulted from antimicrobial resistance-related events generally, out of which 1.27 million were attributable to AR. Six bacteria strains, mostly belonging to the Enterobacteriaceae (otherwise referred to as superbugs) are implicated in most cases. They are Acinetobacter baumannii, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus pneumonia, contributing to their enlistment among the pathogens list of Priority 1 by the World Health Organization. Worrisomely, a forecast of 10 million annual deaths by 2050 has been documented on the menace and more resistant strains with higher lethality could emerge if left unchecked. Thus, concerted scientific efforts are critically required to unveil the pathogenesis of the superbug-inclined AR expansively and strategise effective approaches for overcoming it.
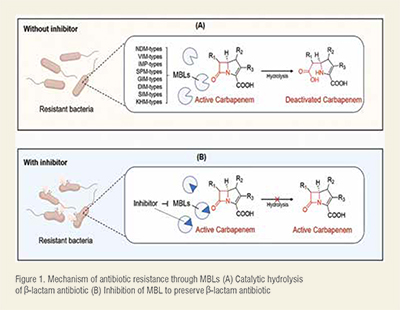
Notably, the production of defensive enzymes such as the metallo-β-lactamases (MBLs) understandably constitutes a major pathogenesis AR among other factors. The New Delhi MBL (NDM), Imipenemase (IMP) and Verona-integron MBL (VIM) are among the most relevant MBLs produced by superbugs for inducing AR. The producer organisms evade the actions of almost all labelled antibiotic medications, including the -lactam/carbapenem groups (even in combined forms) that are commonly prescribed in chronic AR-related clinical conditions. They execute this minacious mechanism by releasing the destructive enzymes, MBLs which in turn damage the integrity of the drugs through a hydrolytic cleavage of their pharmacophoric-lactam rings, facilitated by the coordination chemistry at their Zn sites (Figure 1A). Once deactivated, the molecule is released from the active site of the enzymes and the targeted organism remains drug-free and debilitating in the host. (Figure 1)
Several efforts are being deployed to mitigate the AR challenge by inhibiting the destructive catalytic activities of the relevant MBLs. Among the effective approaches, targeting the Zn ions cofactor of the MBL sites has proved relevant since their enzymatic activities are Zn-dependent. Thus, the strategic chelation of the active site Zn ions through coordination chemistry with small molecules (acting as ligands) (Figure 1B) has attracted an array of scientific interests. Through the fascinating technique, coordinating small molecules reactively sequestrate the Zn ions from the MBL active sites, making the metal less available and distorting the enzymatic activities. Some of the small molecules are potent antibiotics as well, supporting their multi-target-directed actions while acting as adjuvants to conventional antibiotics. Either way, the integrity of antibiotics is protected/restored and the MBLs become inhibited, resulting in an effective mitigation of AR-related cases. More interestingly, some of the reported small molecules have demonstrated excellent inhibitory potencies against multiple strains of the MBL. Such molecules are regarded as broad-spectrum inhibitors and the potent ones with minimal toxicity reported in recent literature are herein overviewed.
The development of effective MBL inhibitors (MBIs) that combine selectivity and low toxicity is a formidable challenge due to shallow grooves and structural diversity characterising MBLs. Researchers' collective efforts have led to the discovery of some promising small-molecule inhibitors with a broad spectrum of action against various MBL subtypes. The progress made in identifying these diverse MBIs offers new hope in combating AR. Few notable ones from both in vitro and pre-clinical in vivo studies are identified below for further translational studies.
Two synthetic molecules, 2,5-dimethyl-4-sulfamoylfuran-3-carboxylic acid (MBI01) and 2,5-diethyl-1-methyl-4-sulfamoylpyrrole-3-carboxylic acid (MBI02), possessing a sulfamoyl heteroarylcarboxylic acid skeleton, exhibited nM-μM range of inhibitory activities on clinically relevant MBL types, including IMP, NDM, and VIM. Additionally, their potencies extend to other types such as the Sao Paulo MBL, Seoul imipenemase, Dutch imipenemase, Tripoli MBL, and Kyorin hospital MBL. These inhibitors bind competitively, weakening the MBL potential for antibiotic deactivation by sequestering the metal cofactors from their active sites. Interestingly, in combination with meropenem, they synergistically reduce the minimum inhibitory concentration (MIC) on various clinical isolates of some MBLs-carrying Enterobacteriaceae up to a hundred-fold. Particularly, MBI02 achieved a feat by raising the survival rate of murine model infected with NDM-1/VIM-1-carrying K. pneumonia clinical strain to an astounding 100 per cent. These findings highlight their potential to be developed for clinical application, especially due to their minimal toxicity.
Another promising candidate, 3-(6-aminopyridin-3-yl)-1-sulfamoyl-1H-pyrrole-2-carboxylic acid (MBI03), synthesized with a pyrrole-carboxylic acid analogue, performed selectively against IMP-1, NDM-1, and VIM-2. When tested in combination with meropenem, it effectively suppressed MBL-producing clinical isolates. Remarkably, the synergism was actively apparent in murine models with resistant bacterial infections. Despite its potent MBL inhibitory action, MBI03 exhibited a remarkable safety profile. It displayed minimal off-target toxicity effects, even beyond the pre-clinical therapeutic doses. This compelling safety profile lays the groundwork for exploring its therapeutic potential with confidence.
In the pursuit of a broad MBL inhibition, di-2-pyridylketone 4-cyclohexyl-4-methyl-3-thiosemicarbazone (MBI04), a synthesised dipyridyl-substituted thio-semicarbazone-based molecule has shown promising results. Its potency remains consistent across the clinically relevant MBLs including IMP-1, NDM-1, and VIM-2, in the range of nM-μM via the formation of Zn-inhibitor complex, indicating a metal-chelating mechanism. When combined with meropenem against IMP-1-, NDM-1-, and VIM-2-producing E. coli at a concentration of just 8 μg mL-1, it exhibited remarkable restorative effects on carbapenem activity, reducing the MIC by up to 256-fold (especially on NDM-1). Encouragingly, in further testing using a carbapenemresistant K. pneumoniae infected murine model, the combination therapy of this inhibitor with the antibiotic significantly reduces the bacterial load. Cell-based assays also validate the safety profile, indicating low cytotoxic even at concentration several times higher than the effective dose. This notable efficacy hints at the potential of this molecule as a valuable addition to the antibiotic arsenal in combating resistant bacterial infections.
In addition, (S)-2-(bis((1H-imidazol-4-yl)methyl)amino)-5-(3-phenyl-5-thioxo-1,5-dihydro-4H-1,2,4- triazol-4-yl) pentanoic acid (MBI05) demonstrated robust targeting actions on NDM- and VIM-type variants. Its mechanism involves a selective interference with the zinc coordination in the MBL active site, resulting in significant inactivation of the enzymes. While it exhibits moderate activity towards IMP-type, its limited zinc accessibility confers greater resilience. Notably, its co-administration alongside meropenem yielded an interesting synergistic effect. They substantially reduced the MIC values by 500 to 1000-fold against NDM-1, VIM-1, and VIM-4 carrying ultra-resistant clinical isolates. The compound stands as a promising candidate due to its minimal off-target toxicity risk, metabolic stability, and reduced cytotoxic potential.
Likewise, 1-(4-(thiophene-2-yl) phenethyl)-1H-imidazole-2-carboxylic acid (MBI06) displayed a broad-spectrum activity against various MBLs. Its most pronounced efficacy, however, is discerned in its ability to selectively target VIM-type MBLs, encompassing VIM-1, -2, and -5 variants. At a minimal concentration (10 μg mL-1), a synergistic interaction with meropenem proved evident. The observed effect can be attributed to its compact molecular size, facilitating facile permeation through bacterial membranes and thereby establishing effective interaction with the Zn active site within MBL enzymes.
The compounds, (R)-6,6'-(((1-carboxyethyl)azanediyl)bis(methylene)) dipicolinic acid (MBI07) and (S)-6,6'-(((1-carboxyethyl)azanediyl) bis(methylene))dipicolinic acid (MBI08) merit attention for their distinctive inhibitory properties. Acting through a non-competitive metal-chelating mechanism, these compounds exhibited a micromolar-level inhibitory effects across a broad spectrum of MBLs, IMP-, NDM-, and VIM-types. In time-kill kinetic assays, co-administration of either of these compounds with meropenem significantly reduced an MBL-inclined bacterial load. Qunatitatively a 10,000-fold reduction in bacterial load carrying IMP-1, a>10,000-fold reduction against NDM-1, and a decrease to <2 CFU mL-1 in the case of VIM-1. Importantly, these compounds displayed a favourable safety profile, worthy of further assessment as future antibiotics.
Beyond the synthetic compounds, nature offers potent alternatives in the form of secondary metabolites or phytochemicals that effectively suppress the MBLs. Noteworthy, emerione A (MBI09), from Aspergillus nidulans, and fisetin(MBI10), found in various vegetables and fruits, stand out as promising candidates. Acting as metal chelators, either through direct interaction with Zn at the MBL enzymatic site or by forming a ternary complex, they inhibit MBLs within the μM range and exhibit independent antibacterial actions against NDM-1-producing Enterobacteriaceae. Co-administration of MBI09 or MBI10 with carbapenem significantly reduced the susceptibility of NDM-1-expressing bacterial strains, and effectively revived the potency of meropenem. In vivo experimentation, the restoration ability of MBI10 is validated in a murine model infected with resistant bacterial strains. Impressively, the two compounds feature ideal systemic safety profiles. Even at acute and chronic high doses, they showed no apparent adverse effects and only insignificant haemolytic effects, positioning them as potential therapeutic agents for addressing MBL-mediated AR.
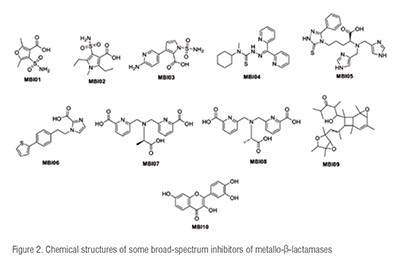
The chemical structures of the ten highlighted MBL inhibitors are presented in Figure 2. Their structural scaffolds support further modification upon structure-activity relationships (SAR), quantitative SAR (QSAR) and other molecular modelling for synthetic derivatization. (Figure 2)
Antibiotic resistance continues to dominate the causes of epidemiology, morbidity and mortality associated with microbial infections. The production of MBL enzymes by the pathogenic bacteria has been implicated in the pathogenesis of AR, affecting the efficiency of almost all antibiotics including the supposedly highly active -lactam types. Thus, the quests for effective MBIs are essentially significant to relevant science fields. From the concerted efforts, researchers have identified some smallmolecules with promising inhibitory effects on the devastating MBLs. Ten of them, MBI01-MBI10 with strong inhibitory activities against various MBL strains have been surveyed from some recently documented in vitro and in vivo experimental validations. These molecules have demonstrated potential for mitigating the Zn-dependent destructive MBL activities inclusively via the mechanistic sequestration of the Zn ions from the MBL active sites. They also displayed a synergistic restoration of antibiotic effects of labelled medications on MBL-carrying organisms and possess ideal safety profiles. Thus, the compounds (eight from synthetic route and two natural products) represent potential broad-spectrum small-molecule inhibitors of MBL amenable for future antibiotics upon further analysis. Although, by targeting and chelating metal ions, concerns have been raised on their potential for off-target inhibition of beneficial metalloenzymes. However, future efforts could be directed at their appropriate delivery to sites of actions suggestively with the aid of nanotechnology. The overview models a template for designing efficacious therapeutics for curbing AR trends upon further translational investigations.
--Issue 53--