This article highlights the relevance of studying brain-enriched miRNAs, the mechanisms underlying their regulation of target gene expression, their dysregulation leading to progressive neurodegeneration, and their potential for biomarker marker and therapeutic intervention. This article has been written to emphasize ways for the effective diagnosis and prevention of these neurodegenerative disorders in the near future.
Neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and Amyotrophic lateral sclerosis (ALS) are a group of age-related progressive disorders initiated by the neuronal loss that eventually leads to cognitive and movement disorders. These diseases are thought to be caused by alterations to protein-coding genes. Non-coding RNAs participate in translational regulation and comprise 95 per cent of total human cellular RNAs (Figure 1)
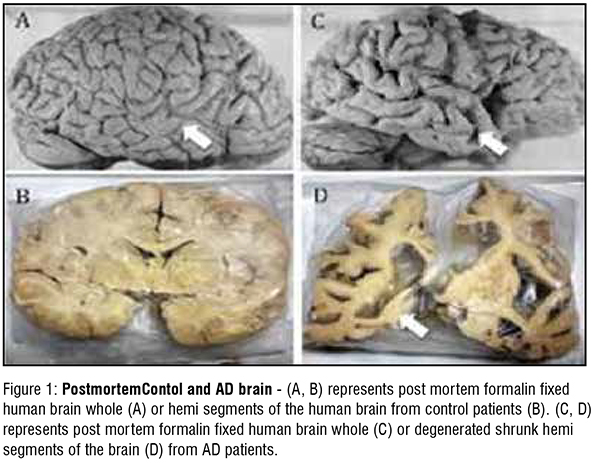
PostmortemContol and AD brain - (A, B) represents post mortem formalin fixed human brain whole (A) or hemi segments of the human brain from control patients (B). (C, D) represents post mortem formalin fixed human brain whole (C) or degenerated shrunk hemi segments of the brain (D) from AD patients.
MicroRNAs are small, non-coding RNA molecules transcribed from RNA polymerase II and III. MicroRNAs are 22 nucleotides long and fixed in the 3' untranslated section. Mature miRNA are formed in the effector complex to act as a post-transcriptional regulator with its target mRNA. Each miRNA comprises of a seed region that is highly conserved and comprises of an area between 2 to 8 nucleotides. This region spans from the 5’ to 3’ end of the miRNA and as a perfect complementarity match with the 3’UTR of the target mRNA.
Extensive research, led to the understanding that small noncoding miRNAs played an important role in fine-tuning the genome. Complex networks in the brain are formed by the usage of transcriptome in wide range of combinations. Modulation of expression of thousands of genes is achieved by specific miRNAs controlling the target mRNA expression, leading to various physiological processes. Primary transcripts of miRNA are synthesised by RNA polymerase from miRNA genes. These transcripts are processed in the nucleus by the Drosha enzyme to produce a hairpin-like precursor miRNA (premiRNA). The pre-miRNA is transported from the nucleus to the cytoplasm by exportin. In the cytoplasm, the premiRNA undergoes shearing by dicer to form the mature miRNA. Ago 1 and Ago2 (Argonaute-1 and Argonaute-2) proteins, mature miRNA containing RISC (RNA induced silencing complex) binds to the 3'UTR of target mRNA. This leads to post-transcriptional inhibition or degradation of the target mRNA. Neuronal degeneration onset begins due to dysregulation in Dicer, Drosha, and RISC complexes leading to disruption of miRNA biogenesis and defective cellular processes (Figure 2)
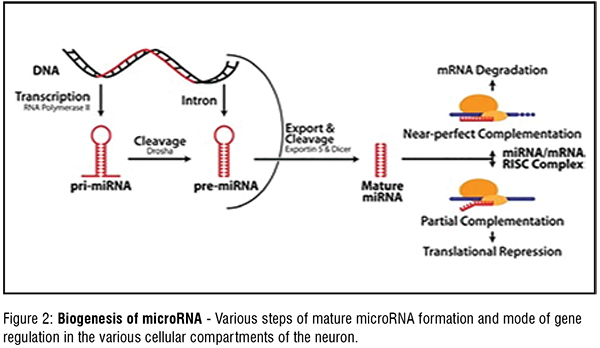
Biogenesis of microRNA -Various steps of mature microRNA formation and mode of gene regulation in the various cellular compartments of the neuron.
Alzheimer’s Disease affects about 60 per cent of age dependent dementia cases amongst elderly people. This neurodegenerative disorder is associated with loss in neuronal tissue, memory, impaired cognitive functioning, and impaired learning. The exact cause of AD is still unknown but prior research shows that there are two biomarkers strongly associated with AD. The first biomarker, tau, is a microtubule-associated protein that promotes vesicle transportation. In AD, hyper phosphorylation of tau causes it to lose its affinity to other molecules. Consequently, this hyper phosphorylated tau develops a stronger affinity for other tau molecules and cause them to adhere together to form Tau aggregates. Increased levels of Tau aggregates lead to a decrease in neuronal communication, due to microtubule instability. Tau is a microtubule binding protein and phosphorylation of Tau lead to its dissociation from the microtubules, thereby destabilizing the microtubule assembly. This eventually lead to defective axonal transport and impaired synaptic transmission across neurons in a neuronal circuit. The second biomarker is amyloid- , which is a product of the APP (amyloid- precursor protein), and is known to form amyloid- plaques in AD patients. Research studies show that there is another biomarker in addition to hyper-phosphorylated Tau and amyloidplaques, known as miRNAs. Several research investigations discuss the presence of miRNAs circulating within the blood and cerebrospinal fluid. Most of these miRNAs have experimentally validated targets, known to be important in regulating various pathological processes in the neurons leading to AD (Figure 3). On the other hand, certain miRNAs have been shown to have altered levels in blood plasma, cerebrospinal fluid, or post- mortem brain tissues in AD patients. Additionally, there are various molecular players modulated by miRNAs in other neurodegenerative diseases like Parkinson’s
Disease (PD), Huntington's Disease (HD) and Amyotrophic Lateral Sclerosis (ALS). The miRNA target gene schematic diagram (Figure 4) highlights some of the important genes whose levels post-transcriptionally modified miRNAs, leading to alteration of various vital cellular functions of the neurons. This eventually leads to their degeneration (Figure 3).
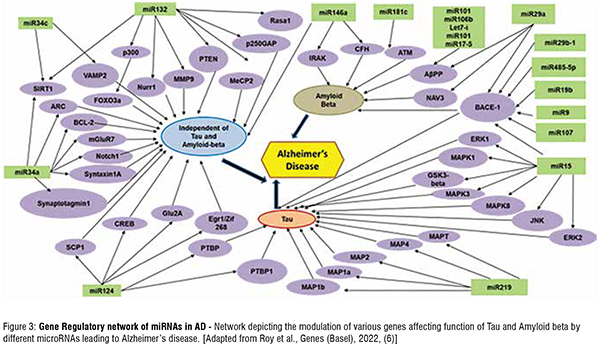
Gene Regulatory network of miRNAs in AD - Network depicting the modulation of various genes affecting function of Tau and Amyloid beta by different microRNAs leading to Alzheimer’s disease. [Adapted from Roy et al., Genes
(Basel), 2022, (6)] (Figure 4).
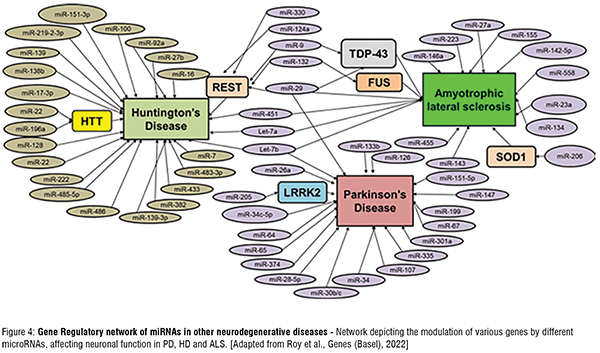
Gene Regulatory network of miRNAs in other neurodegenerative diseases - Network depicting the modulation of various genes by different microRNAs, affecting neuronal function in PD, HD and ALS. [Adapted from Roy et al., Genes (Basel), 2022]
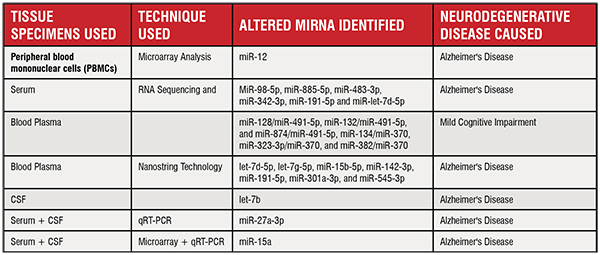
Molecular, genetic and biochemical components that aid in the identification and analysis of pathological processes in the human body are referred to as biomarkers. They can be used as tools to assess the different stages of diseases, especially the early or preclinical stages. Detection of biomarkers in the early stage of any disease may enable patients to receive early therapy. MicroRNA has been advocated as a possible biomarker for different types of diseases regarding diagnosis and treatment. These diseases include, neurodegenerative diseases such as AD and different types of cancer (lung carcinoma, gastric cancer, oesophageal cancer, breast cancer, colorectal cancer). A widespread methodology used in miRNA biomarker study is the utilisation of their levels in the cerebrospinal fluid (CSF) as a minimally invasive detection tool for preclinical markers for central nervous system diseases like AD, lymphomas and gliomas. Circulating miRNAs in the body fluids, showing statistically significant alteration levels, have been widely accepted as an important diagnostic factor in miRNA biomarker research in many diseases, including AD. Several research groups used different human patient samples (serum, peripheral blood mononuclear cells, cerebrospinal fluid) and different techniques (to measure circulating microRNAs) (Figure 5).

Table listing the miRNAs found as potential biomarkers in Alzheimer’s disease– The table lists the various techniques and tissue samples used for measuring miRNA levels in AD patient samples and can serve as potential strategies for biomarker studies in clinical diagnostics.
Usage of CSF samples can be a useful methodology for detecting circulating disease specific or associated miRNAs. Despite few ongoing studies, further research and more easily viable, cost effective and user friendly applications or methodologies are needed for implementing miRNAs as biomarkers for AD. The application of CSF to detect miRNAs is highly recommended in comparison to blood. Blood samples are easily obtained at a low-cost and low risk. However, the molecular profile generated from blood and CSF samples can vary, as there is a possibility of additional factors being found in the blood (which are absent from the brain), due to blood’s systemic circulation properties. Cerebrospinal fluid, on the other hand might be a more reliable biomarker, because it is protected by the blood brain barrier, which reduces unreliable biological factors that may influence the miRNA composition in AD or any other brain disorder. Internal and external factors influencing miRNA levels, include genetic variation, age, gender, race, inflammatory status, lifestyle, methodologies or techniques used to process samples and measure miRNA levels. MicroRNA concentration levels may vary in serum and plasma samples, within the same person. Research study have shown that different types and concentrations of miRNA were found in blood, plasma, serum and exosome samples. Thus, a careful analysis of data from these various samples should be done as a downstream processing protocol in every biomarker study. Additionally, precaution should be taken in the process of analysing the downstream data, for understanding the statistical significance and the relevance of the data in biomarker studies. The variation of miRNA measurement requires a more standardised protocol, such as consistent sample preparation, statistical calculations, and systematic analysis of unvarying miRNA levels. In summary, miRNA biomarker research has the potential in understanding the pathogenesis of these age related neurodegenerative disease and deciphering the underlying regulatory molecular mechanisms leading to their clinical manifestations. As a step forward, miRNA biomarker research in a more detailed and comprehensive manner might help us to monitor and diagnose these diseases at the earlier pre-clinical stage.
Overview: Delivery of miRNA into the central nervous system, is very challenging due to the presence of blood brain barrier (BBB). The BBB prevents the accumulation of bio-active compounds in the CNS, thereby posing as a limitation for transfection efficiency for miRNA mimics or miRNA inhibitors introduction. To increase the transfection efficiency of these miRNAs and as an aid in their blood- brain barrier crossing, two strategies have been formulated. In the first strategy, the restoration of the suppressed miRNA is achieved by introduction miRNA mimics (agonists). In the second method, the miRNA levels are lowered by inhibiting their activity by using anti-miR agents (antagonists). Depending on the therapeutic requirement, these strategic methods of either, increasing the expression of miRNA or lowering their levels, can be used effectively to modulate the levels of disease specific miRNAs in ameliorating the clinical manifestations of these neurodegenerative diseases. The methods required to suppress the function of microRNAs are anti-miRs oligonucleotide (AMOs), antagomirs, miRNA sponges, locked nucleic acids (LNA) anti-miRs and miR-masks. The methods required to enhance the activity or expression levels of miRNAs are by inducing miRNA expression vectors and miRNA mimics. Therapeutic application of miRNAs-based agents poses towards an encouraging and optimistic path towards the initiative of finding a cure for various neurodegenerative disorders, including the ones rooted deep in the central nervous system, due to these following properties: easier manufacturing methods, bio-safety, simplicity, potency and effectiveness and optimum duration of gene expression silencing. Within the context of brain delivery, the distribution of miRNAbased therapeutics and their effectiveness can also be influenced by the route of delivery. To overcome this issue, two methods have been applied for drug transport across the BBB, namely invasive and non-invasive approaches. The invasive approaches include Intra cerebro-ventricular infusion, intrathecal, convection-enhanced delivery, and the disruption methods of BBB integrity. The non-invasive approaches include lipid-mediated drug transport, systemic intravenous administration, intranasal delivery, and strategies using nano- systems.
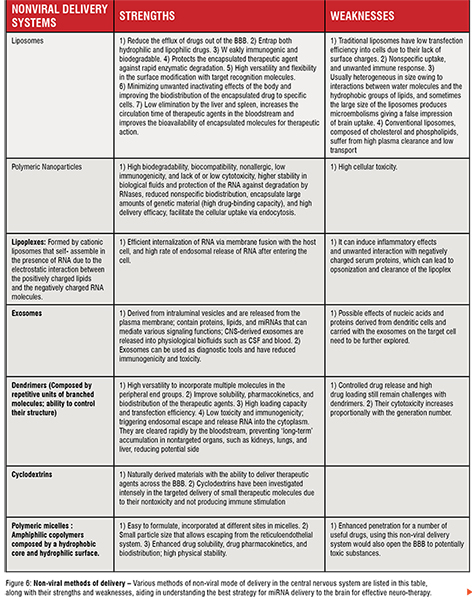
Different and efficient carrier systems have been developed and applied in gene therapy trials to promote the transport and delivery of miRNAsbased therapeutics into the brain, increasing their accumulation in the site of interest, enhancing the silencing potency, thereby making these kinds of RNA therapeutics more effective. In a simple way, delivery methods can be divided into two categories, non-viral (Figure 6) and viral systems (Figure 7).
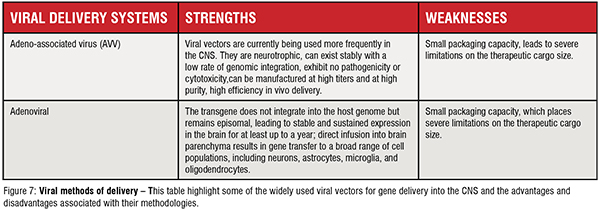
Viral methods of delivery – This table highlight some of the widely used viral vectors for gene delivery into the CNS and the advantages and disadvantages associated with their methodologies.
RNA-based therapeutics is emerging as potential drugs in various clinical fields, including neurodegeneration. RNA based therapeutics have yielded extremely positive results. However, these results do not include miRNA - based therapeutic strategies targeting against AD pathology. Patisiran, the first siRNA drug was approved by the FDA in 2018 for the treatment of hereditary transthyretin-mediated amyloidosis. In this methodology, siRNA was packaged in lipid nanoparticles and directed to the liver, where it targeted the mRNA of transthyretin and blocked the production of the mutant protein. Treatment for spinal muscular atrophy (SMA) was approved in 2016 using a 2 -O-2-methoxyethyl phosphorothioate-modified Antisense oligonucleotide (ASO). Splicing of SMN2 mRNA was inhibited by the ASO, thereby increasing the amount of functional SMN2 protein, which could rescue the loss of SMN1 phenotype by compensating for the loss of SMN1 Another RNA-based therapy (zolgensma) was approved by the FDA for SMA, where a functional copy of SMN1 gene is delivered using an adenoviral AAV9 delivery system. Translational inhibition of tau mRNA using an ASO-based strategy (NCT03186989, BIIB080, IONISMAPTRX), is currently in a clinical phase I/II trial study. With respect to the miRNA-targeted pharmaceutical gallery, numerous ongoing clinical trials (CDR132L, Cardior Pharmaceuticals GmbH; RG012, Genzyme /Sanofi / Regulus Therapeutics; MRG-106, MRG-110, MRG-201, miRagen / Viridian Therapeutics; TargomiRs) are being continued. However, none of these trials are targeted for AD therapy. In the spectrum of neurodegenerative disorder, MRG-107, a miR-155 inhibitor has been pre-clinically tested and validated by miRagen Therapeutics against ALS.
Till now, even though various clinical trials are ongoing to test miRNAbased therapeutics against several peripheral diseases, none have been attributed for the treatment of AD. However, gemfibrozil, a previously FDA-approved drug for decreasing cholesterol and lipids, has undergone a phase I trial to evaluate its ability to increase miR-107 levels for the prevention of AD in cognitive health and MCI individuals (NCT02045056). Gemfibrozil was found to be a safe drug, in inducing a change in miR-107 plasma levels, decreasing A 42, pTAU, A 42/ pTau ratio, brain atrophy, and plasma TNF levels in the treated patients. However, these studies did not reach a statistical significance. MiRNA-based therapeutics is a developing field in comparison to other oligonucleotide based therapeutics, like siRNA and ASO. Therefore it is essential to conduct a more detailed, comprehensive basic research to characterise how iRNAs target molecular and cellular pathways in neurodegenerative diseases. Additionally, a systematic mapping of the on- and off- target toxic effects of miRNA based gene regulation should also be considered while designing miRNA based therapies. These factors serve as important prerequisites for effective clinical application of miRNA in treatment of AD and other neurodegenerative disorders. Additionally, better methods of targeted brain delivery and additional investigation of the tolerability of miRNA restoration strategies are key issues to be resolved. Even though a large number of miRNA-based companies are being acquired by major pharmaceutical companies to help find a novel category of drugs, the application of miRNA therapeutics in AD is lagging behind other diseases areas such as cancer, which has approximately 30 times more new molecular targets in clinical trials than AD. Technology improvisation, aggressive monetary investment, and research development in the field of neurodegenerative disorders such as AD, are needed to bridge the gap between promising initial bench side miRNA research and clinical application.
References:
1. Pearson H. Genetics: what is a gene? Nature. 2006;441(7092):398-401.
2. Lagos-Quintana M, Rauhut R, Lendeckel W, and Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853- 8.
3. Kapsimali M, Kloosterman WP, de Bruijn E, Rosa F, Plasterk RH, and Wilson SW. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 2007;8(8):R173.
4. Hebert SS, Sergeant N, and Buee L. MicroRNAs and the Regulation of Tau Metabolism. Int J Alzheimers Dis. 2012;2012:406561.
5. Pan Y, Liu R, Terpstra E, Wang Y, Qiao F, Wang J, et al. Dysregulation and diagnostic potential of microRNA in Alzheimer's disease. J Alzheimers Dis. 2016;49(1):1-12.
6. Roy B, Lee E, Li T, and Rampersaud M. Role of miRNAs in Neurodegeneration: From Disease Cause to Tools of Biomarker Discovery and Therapeutics. Genes (Basel). 2022;13(3).
7. Cheng L, Sharples RA, Scicluna BJ, and Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014;3.