Despite the recent advancement in cancer therapy, the death rate of cancer is increasing every day. Stem cell therapy emerged as highly successful therapy for several cancers. In recent years, the stem cell manipulation and development of induced pluripotent insistent cells have shown tremendous potential to treat cancer.
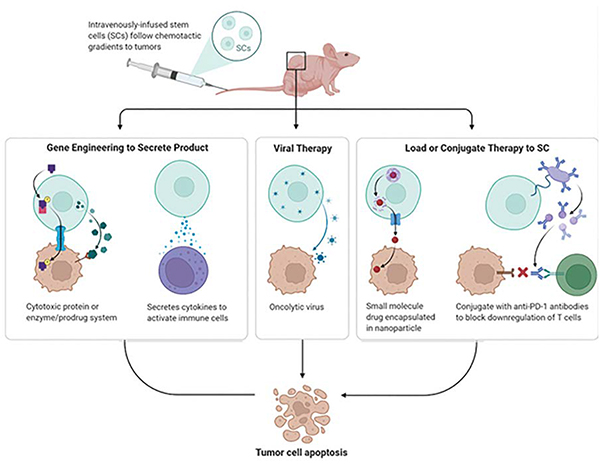
Despite several advances research cancer remains one of the deadliest diseases across the world. The type of treatment provided depends upon the type and progression of cancer. Surgery is the first option recommended to remove solid tumours from a single area. Radiation, chemotherapy, cancer vaccines remain some of the other options to choose to slow down or stop tumour growth. However, stem cell therapy has provided new hopes in this fight. It could possibly improve the therapeutic efficacy due to its enhanced specific target on tumours. Metastatic cancer cells generally cannot be eradicated using traditional surgical or chemoradio therapeutic strategies, and disease recurrence is extremely common following treatment. However, therapies involving stem cells show increasing promise in the treatment of cancer. Stem cells can function as novel delivery platforms by having the ability to target both primary and metastatic tumour foci. Stem cells engineered to stably express various cytotoxic agents decrease tumour volumes and extend survival in preclinical animal models. They have also been employed as virus and nanoparticle carriers to enhance primary therapeutic efficacies and relieve treatment side effects. Stem cells have unique properties, such as migration toward cancer cells, secretion of bioactive factors, and immune suppression, which promote tumour targeting and circumvent obstacles currently impeding gene therapy strategies. Preclinical stem cell-based strategies show great promise for use in targeted anti-cancer therapy applications. In addition to their self-renewal and differentiation capabilities, stem cells have immunosuppressive, anti-tumour, and migratory properties. Because stem cells express growth factors and cytokines that regulate host innate and cellular immune pathways, they can be manipulated to both escape the host immune response and act as cellular delivery agents. Stem cells can also secret factors, such as CCL2/MCP-1, and physically interact with tumour cells, changing co-cultured tumour cell phenotypes and exerting intrinsic anti-tumour effects. Stem cells, most commonly neural stem cells (NSCs) and mesenchymal stem cells (MSCs), can be modified via multiple mechanisms for potential use in cancer therapies. Common modifications include the therapeutic enzyme/prodrug system, and nanoparticle or oncolytic virus delivery at the tumour site.

NSCs and MSCs can be designed to express enzymes that transform non-toxic drugs into cytotoxic products. When modified stem cells are transplanted to tumour models, they are positioned in tumour tissue. The exogenous enzymes convert the prodrug into cytotoxic molecules, ultimately damaging tumour cells. As a result, the amount, timing, and location of drug release can be precisely controlled. Enzyme and pharmaceutical therapy is also known as suicide genetic therapy, and is the first therapeutic application developed in NSC and the first to be introduced into clinical trials. Stem cells can work as in situ drug factories and secrete long-term anti-tumour agents, and can overcome various cancer therapy limitations, such as high systematic toxicity and short drug half-life. TNF- -related apoptosisinducing ligand (TRAIL) is one of the most widely used, secreted therapeutic agents, and induces tumour cell apoptosis. However, its short half-life reduces its therapeutic effectiveness in vivo. Stem cells can also be modified to selectively deliver growth inhibitory proteins (e.g., IFN- ), rendering the microenvironment inhospitable to tumour growth. Ling, et al. studied the migration of IFN-expressing MSCs and their engraftment into primary breast tumour sites, and found that tumour cell growth was suppressed, and hepatic and pulmonary metastases were alleviated. Oncolytic viruses (OVs), unlike traditional attenuated viruses, conditionally replicate in tumour cells. OVs have increased spread in the body and hide from the immune system. OV-transduced NSCs are still able to home to tumour cells, and NSC-delivered OVs showed better antitumour effects than the viruses alone against GBMs in vivo. Virus delivery by MSCs is also a promising approach for targeted cancer therapy. Ong, et al. demonstrated that the potent oncolytic activity of attenuated measles virus combined with the unique immunoprivileged and tumour-tropic properties of MSCs could combat hepatocellular carcinoma.
As stem cells have the ability to self-renew and differentiate, they can be used to repair human tissues after chemotherapy. HSC transplantation has been widely used in clinical practice to facilitate life-long hematological recovery after high dose radiotherapy or chemotherapy treatment of malignant patients. This treatment is aimed at reconstructing bone marrow under conditions of bone marrow failure (e.g., aplastic anaemia) and treating genetic diseases of blood cells, and works by supplying stem cells that differentiate into a desired type of blood cell. The transplantation and successful transplantation of a single HSC can restore hemopoiesis to the recipients. Patientspecific iPSCs could also potentially benefit immunotherapy approaches. The pre-rearranged TCR gene is retained in T lymphocyte-derived human iPSCs, which can be further induced to differentiate into functionally active T cells. Functional T lymphocytes, specific to the tumour antigen, can be produced in vitro by reprogramming selected T cells into iPSCs and then differentiated again into T lymphocytes for infusion in patients. However, the safety of T cellderived human iPSCs must be further validated.
Tumours commonly relapse regardless of strong initial therapeutic effects. Like most chemotherapy, the use of singlemolecule stem cell therapy is generally not capable of eliminating tumours. Consequently, it is necessary to select the optimum combination of drugs rationally. Many combinations of therapy have been tested to improve the long-term effectiveness of treatment. For example, combined with antigen/suicide genes, immunotherapy using IFN- antibody has shown a synergistic therapeutic effect on human colorectal cancer. Irradiated tumour cells can induce the production of factors that stimulate the invasion of the SMC by the whole membrane of the soil, which increases the number of the SMC in the tumour. Combining stem cell-based immunotherapy and chemical radiotherapy can minimise the residual volume of the disease and give glioma cells a CRAd-S-pk7 (OV CRAd-Survivin-pk7) sensitivity during radiotherapy. Kim and colleagues found that TMZ stimulated glioma cells to apoptosis induced by TRAIL by modulating apoptotic machinery and improving MSC-TRAIL gene therapy’s anti-tumour effect. Epidermal growth factor receptors (EGFRs) mutated and exaggerated in several tumours have low prognosis and reduced survival rates. TRAIL and the immune conjugation of stem cells of nanobodies specific to EGFR have improved the results of treatment. The risk profile of stem cell based medicinal products depends on many risk factors, which include the type of stem cells, their differentiation status and proliferation capacity, the route of administration, the intended location, in vitro culture and/or other manipulation steps, irreversibility of treatment, need/possibility for concurrent tissue regeneration in case of irreversible tissue loss, and long-term survival of engrafted cells. Together these factors determine the risk profile associated with a stem cell based medicinal product. The identified risks (i.e. risks identified in clinical experience) or potential/theoretical risks (i.e. risks observed in animal studies) include tumour formation, unwanted immune responses and the transmission of adventitious agents.