Personalised medicine breaks the cycle of trial and error medicine and helps ineffectiveness rates of medicines to decrease dramatically. Orphan drugs, targeted therapies for rare diseases, with their innovative nature and improved efficacy, make it a precursor for personalised medicine therapies and can offer a viable business model to expand from. Society should develop consensus for such new models in multi-stakeholder partnerships which will also make it sustainable.
For many rare diseases, there is as yet no satisfactory treatment. Rare diseases are defined as life-threatening and / or serious and chronic diseases, according to the European Orphan Medicinal Products regulation. These diseases represent a high unmet medical need. Rare diseases, with their genetic origin and because of small number of patients to be treated, require effective diagnosis linked to treatment. So, orphan drugs, with their targeted nature, are of immense use and could pave the way for future developments in healthcare
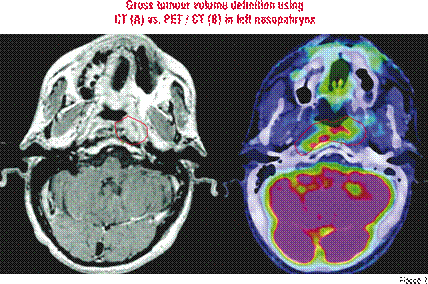
Since 70 to 80 per cent of rare diseases are genetic in origin, the right diagnosis before treatment is of high importance to ensure that the patient not only gets the right treatment but also does not suffer from side effects. The rarity and severity of the diseases not only mean lesser patients are treated but also result in a high compliance because the medicines work. Rarity also leads to higher costs, but orphan drugs also have a higher innovative nature and a higher benefit than average drugs through their better clinical efficacy and effectiveness. In the European definition, orphan drugs are unique treatments, meaning there are no alternatives available. This is the case for about one-third of the orphan drugs approved in Europe) or make a better therapy over (an) existing one(s), which is the case for the other two-thirds of approved orphan drugs in Europe. Not only economic factors but social benefits also need to be taken into account as well: many patients affected by rare diseases had no therapy available before and such therapy is often a first in human history.
Innovation, rarity and complexities caused
The current debate in society is about the value of innovation in healthcare: our society supports such beneficial or valuable innovation to the benefit of patients, including those in the case of orphan drugs. Even in difficult economic times, such beneficial innovation must be encouraged and the resulting products must be reimbursed when they have measurable and demonstrable value. However, given the rarity of the diseases studied, sufficient time to gather data to show such value must be granted.
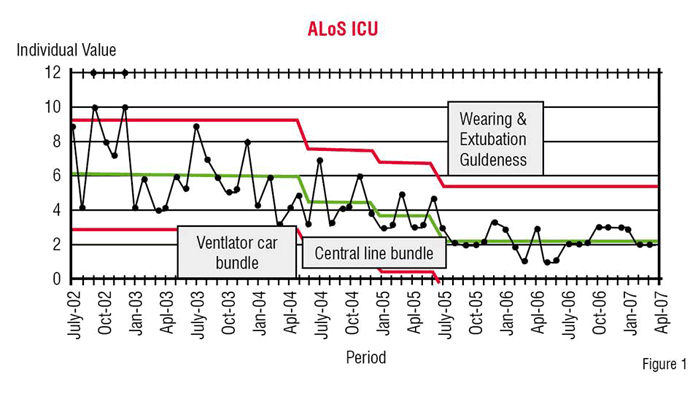
In order to understand the concept of rarity, Figures 1 and 2 provide the number of patients treated in the US in 2006 for a number of non-rare disease and, from Gleevec (Glivec TM) downwards, for rare diseases.
The application of models used in health economics introduces further challenges to the development of orphan medicines and to the discussion about their reimbursement.
Cost-Effectiveness = Cost / Effectiveness, where cost is raised by rarity, but data to calculate effectiveness are limited by rarity. Therefore, rarity is affecting both the factors of the equation making the outcome much more uncertain as it grows.
Based on this, it has to be clear that cost-effectiveness cannot be the only decisive factor. Current definitions of health technology assessment, therefore, include other factors such as equity, fairness, ethics and social values. Politicians need to take responsibility to reward innovations in this field to allow them to happen and to continue.
As shown by the consultancy company Alcimed in a report for the European commission in 2004, pricing is a function of the rarity of the treated disease. Therefore, the prices of orphan drugs, given that their development cost is not necessarily lower than the cost to develop a medicine for a common disease, will be higher than the prices of drugs for treating a (more) common disease. As such, prices largely depend on the patient population to be treated. As a consequence, the application of the same rules of cost-effectiveness for orphan drugs, and especially for drugs for very rare diseases, will not be appropriate, as this would result in orphan drugs and even more clearly the so-called ultra-orphan drugs being excluded from reimbursement since they often do not reach the required Quality Adjusted Life Year (QALY) limit.
The future of personalised medicine
Further, drawing the analogy with personalised medicine, a future with more compliance and far less side effects would be the goal. This will ultimately result in costs going down, as only the right medicine would reach the right patient and minimise side effects. In order to do that, the following series of questions need to be answered:
The old paradigm of trial and error medicine, based on the sequence of an observation with an action followed by an observable response is only successful when it leads to innovation and improved standard of care. But it fails when we settle for “trial and error” medicine as the standard of care. The new paradigm of personalised medicine links diagnostic tests to action and therapy. This happens by having an observation confirmed by a test and have it followed by appropriate physician actions which will result in a predictable response, that breaks the cycle of trial and error medicine and helps the patient. Personalised medicine leads to the right drug for the right patient. It results in providing benefits without toxicity. It saves patients from the treatment that would have the benefits but with the toxicity of the medicine if any. Also, it excludes them from the treatments that would have no benefits and no toxicity, and, of course, also from those without any benefits but with toxicity.
As shown in Figure 3, the current ineffectiveness rate of medicines is ranging from 40 to 75 per cent, but this percentage will dramatically decrease with the venue of personalised medicine.
No future without challenges
The current evolution in evidence-based medicine also comes with challenges:
The only solution to such complex societal problems and in order to build new healthcare systems for the future is to be found by the creation of multi-stakeholder partnerships to develop consensus on the way forward. This should result in a wheel of sustainability: life-saving treatments provide a better patient outcome, which warrants reimbursement, and as a consequence, rewards risk-taking and provides return on investment, which in turn leads to more investment in risky projects, resulting in more effective treatments coming onto the market. Clearly, having a market for personalised medicines is in the society’s best interest. We must all work on making it happen!
As the public image of the pharmaceutical industry is challenging, better and clearer communication is needed to explain the contributions it makes to society. And while no company on its own can change the sector’s image, industry must work together. Personalised medicine represents a great opportunity to change public image of the industry for the better!