The Pharmaceutical Industry is not transforming fast enough to address the persistent decline in productivity. Alternative drug development models are being explored in an effort to jumpstart transformation. Eli Lilly and Company has created and successfully implemented Chorus, an autonomous early phase drug development group operating on a fully outsourced model to cost-effectively advance candidate molecules from discovery through clinical Proof of Concept.
In a recent article in Scrip, Anju Ghangurde reported on Eli Lilly and Company’s efforts to effect transformation from a Fully Integrated Pharmaceutical Company (FIPCo) to a Fully Integrated Pharmaceutical Network (FIPNet). The foundational thinking in the move to FIPNet is simply that the current pharmaceutical development process is broken and does not meet today’s market demands; the square block won’t fit into the circular hole… no matter how big your hammer is. This call for transformation is not unique to Eli Lilly and Company but a herald throughout industry. Describing developments in medicine, current head of the U.S. Food and Drug Administration, Andrew Von Eschenbach recently stated, “ The transformation is not a linear extrapolation of the past. It’s a metamorphosis. The future will look no more like the past than a butterfly looks like a caterpillar.” With the ever rising cost of drug development coupled to the apparent dearth of new molecular entities entering the marketplace, it would appear that transformation is not occurring fast enough. To borrow Dr Von Eschenbach’s analogy, the pharmaceutical industry has too many caterpillars.
Transformation to FIPNet – A success story
With the need for transformation in mind, in 2002 Eli Lilly and Company created Chorus via its eLilly initiative. Chorus is a collection of experienced individuals from pharmaceutical industry that functions as an autonomous early phase drug development group empowered to quickly and cost-effectively advance candidate molecules from discovery through clinical Proof of Concept (PoC). A fully functional example of FIPNet, Chorus has adopted a fully outsourced model in which work product gets completed via a network of Third Party Providers (TPP), external consultants, and internal core expertise. Chorus has grown and evolved considerably. Six years into existence, Chorus has taken more than two dozen assets into development from discovery to clinical PoC at a mean cycle time and cost per asset of just under 32 months and US$ 4.5 million, respectively. Data from a 2000 Tufts report place the cost of Phase 1 development without getting to a PoC at US$ 15.2 million. In a subsequent analysis published in early 2006, Adams and Brantner place the mean cost of Phase 1 development at US$ 32 million.
Chorus strategically embraces three core ideals:
1. Increase efficiency over traditional drug development—Lower cost, faster cycle times to drive higher productivity;
2. Faster, more effective decision-making to drive rapidly to value inflection and an earlier investment decision point; and
3. Limit parallel processing and gate resource utilisation pending discharge of target and molecule risk (i.e. execute a development plan that discharges key risks early at low cost to drive a large change in technical probability of success for a modest investment).
Today, Chorus manages a significant percentage of Lilly’s early phase portfolio with a multi-functional research and development infrastructure consisting of approximately 30 internal experienced drug development experts. Individuals are responsible for the core functions of Medical, Regulatory, Toxicology, ADME, CM&C, Clinical Operations, Statistics and Quality. Chorus’ small size and flat structure has multiple benefits. First, the minimal infrastructure allows for a low operating cost. The ratio of fixed versus flexible development costs is more favourable for Chorus when compared to traditional FIPCo infrastructure. Second, it allows for quick decision-making and promotes efficiency in seeking new data or reacting to incoming data. Lastly, it fosters an “act like an owner” culture and functional ownership for delivering lean experimental plans designed to directly contribute to the next decision point in each asset’s development plan. As such, the focus is on optimising the molecule and not the functional infrastructure of traditional FIPNets.
Chorus’ external network consists of approximately 30 external consultants and over 200 global TPPs. With a fully outsourcing model, TPPs are considered an integral part of the team (a virtual project team member). Therefore, Chorus only selects TPPs that can work independently, demonstrate and maintain a track record for on-time milestone delivery, function well with compressed timelines, and bring creativity and innovation to the team. Whenever possible, Chorus uses TPPs’ processes, tools, and templates to minimise imposed sponsor burdens to its partners. To aid in effective global, virtual collaboration, Chorus utilises Voice©, a proprietary, integrated IT system of custom and packaged applications, developed by Chorus in collaboration with an external consulting company exclusively for the pharmaceutical industry. Voice© enables small, virtual teams to globally interact and collaborate on pharmaceutical drug development plans and priorities. Voice© conveys accountability and leverages defined processes to integrate information across a development plan to facilitate real-time planning and quicker strategic decision-making.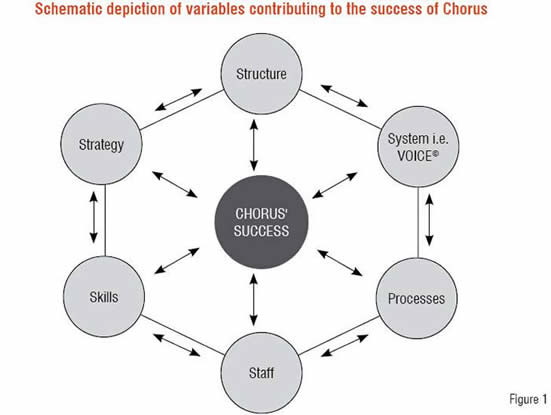
Integrated Decisions
Chorus’ strategic focus, autonomy, structure, streamlined SOPs and business processes, experienced personnel, skilled TPPs, and innovative IT system Voice© interplay in decision-making and contribute to its success (Figure 1). Strategic short and long-term goals are set for each project plan to ensure optimal productivity. Factors such as disease prevalence by geography, subject / patient availability, TPP expertise, regulatory environment, investigator expertise, and standards of care are discussed and vetted as a functional collaboration. Final operational plans are determined through these collaborative discussions, relying on quality, speed, and innovation as the key driving factors.
Chorus country and site selection illustrates this dynamic. A survey of Clinicaltrials.gov shows that 56 per cent of the clinical trials are currently being conducted in the United States, while approximately 23 per cent are being conducted in Europe. Competition for subjects / patients in these geographies can be fierce and plays an important role in slow recruitment time. While arguably the US and Europe offer the greatest experience in conducting clinical trials, Chorus has found multiple Clinical Research Units (CRUs) of excellent quality in other countries to conduct their early phase clinical trials. These CRUs are often headed by physicians and clinical pharmacologists who have been trained in the US or Europe, practiced in these countries for a significant time, and who have returned to their native countries to continue their research. Tapping into these experienced investigators often provides real, tangible benefits for a drug development organisation. The challenges in the process include: difficult to find patient populations (e.g., biologic naïve patients, rare tumours or diseases, etc.), high quality, GCP trained CRUs, decreased trial costs, and in some instances faster regulatory review timelines. A Chorus cross-functional team weighs these operational challenges with their own functional strategy in arriving at the overall drug development plan including country and site selection. Thus, Chorus operates globally in multiple geographies that offer clinical expertise beyond traditional Phase 1 and Phase 2a locales. While these non-traditional countries may not be appropriate for every molecule or development plan, they are selected as a result of carefully considered, integrated decision-making and consequently produce, with rare exception, resounding success.
In conclusion
Although the Pharmaceutical Industry may not be transforming fast enough, Chorus’ “out of the gate” initiative is producing tangible results. Chorus’ alternative drug development model, has utilised a full outsourced model and is cost-effectively advancing molecules from discovery through clinical PoC. Over six years into existence, Chorus has successfully implemented over two dozen development plans at a fraction of the traditional cost utilising a FIPNet strategy. To further illustrate Lilly’s confidence in the Chorus drug development model, Lilly has recently announced the formation of an equally-owned joint venture with Jubilant Organosys to be located in India that will focus on providing drug development services exclusively to Jubilant- and Lilly-partnered molecules. The joint venture, known as Vanthys and based out of Bangalore, is modelled after Chorus, and will have the goal of providing fast and capable drug development for Lilly and Jubilant through the utilisation of external contract companies.
As additional alternative early development models are contemplated, there are several key learnings that have contributed to Chorus’ substantial productivity improvements and overall success that may be worthy of consideration. These include but are not limited to:
• Get to the key studies that have maximal risk as rapidly as possible, minimising parallel processing until data supports an investment decision
• Streamline SOPs and business processes
• Staff with experienced drug developers
• Minimise governance, maximise leadership accountability
• Provide sufficient autonomy to facilitate rapid decision-making
• Provide budgetary, sourcing, procurement and contracting authority to create an “act like an owner” culture
• Consider TPPs an integral part of the team with decision-making authority
• Utilise TPPs processes, tools, and templates when possible
• Integrate functional strategies into long-term molecule development planning
• Invest in technology to assist in effective communication and collaboration in a virtual environment.
As the pharmaceutical industry transforms its business practices, it will be interesting to see how many and what variety of butterflies are produced.
References
1. Ghangurde A. Lilly shifts to “FIPNet” to improve productivity and access to assets. SCRIP World Pharmaceutical News. 24 October 2008.
2. Hill M. Medical Advances will alter the FDA: the agency’s chief says medical-device, drug and biotech makers will be forced to work together. Philadelphia Inquirer. http://www.philly.com/philly/business/20081108_Medical_advances_will_alter_the_FDA.html?adString=ph.business/business;!category=business;&randomOrd=111408123244. Accessed 14 November 2008.
3. Longman R. 2007. Lilly’s Chorus experiment. IN VIVO: The Business & Medicine Report. 25(5):1-5.
4. DiMasi JA, Hansen RW, Grabowski HG. 2003. The price of innovation: new estimates of drug development costs. J Health Econ. 22:151-85.
5. Adams CP, Brantner VV. 2006. Estimating The Cost Of New Drug Development: Is It Really $802 Million? Health Affairs 25(2): 420–428.
6. http://www.ClinicalTrials.gov. Accessed 14 November 2008.
7. Jubilant Organosys and Lilly to Form Drug Development Joint Venture: Indian J.V. expands companies' drug discovery and development collaboration. http://jubl.com/news_desc.jsp?news=177&hl=NH. News Release. 3 October 2008.