In some groups, like kidney transplant recipients, the efficacy of COVID-19 vaccines has been questioned. The sera of COVID-19 vaccinated young individuals are most likely a feasible method for illness prevention and early treatment in such patients. To assess the risk/benefit ratio, clinical studies should be conducted.

Coronavirus disease 2019 (COVID-19) is a worldwide pandemic caused by SARSCoV-2, a highly contagious respiratory virus. As of the end of August 2021, more than 200 million cases have been recorded and led to more than 4 million deaths. Advanced age, diabetes mellitus, cancer, cardiovascular diseases, autoimmune diseases are among many other risk factors for developing severe COVID-19 infection as well as increased mortality rate. Several vaccines were developed and approved for global use with good efficacy and safety in the general population. However, there is a concern of reduced efficacy in the special population such as kidney transplant patients (KTP). Given the negative impact of immunosuppressive drugs on immune response, the efficacy of COVID-19 vaccines is likely to be reduced in KTP patients. There have been some reports of KTP limited early antibody response upon receiving the vaccination.
The majority of repurposed drugs for the management of COVID-19 exhibited limitations. Approximately 30 per cent of the possible COVID-19 medications are supported by moderate or high confidence. For example, lack of efficacy of lopinavir; conflicting results of hydroxychloroquine and so forth. The FDA-approved drug, Remdesivir, is limited by its high cost, need for hospitalisation, and potentially serious adverse effects. The WHO Solidarity Trial conducted among the hospitalised COVID-19 patient revealed that the following drugs: Remdesivir, Hydroxychloroquine, Lopinavir/Ritonavir, and Interferon; did not have a significant effect on total mortality, ventilation initiation, or length of hospitalisation compared to the standard treatment. Furthermore, limited access to intensive care unit (ICU), shortage of oxygen supply, complicated the management plans especially in low or moderate-income countries.
Neutralising monoclonal antibodies against SARS-CoV-2 (NMAB) is immediate and passive immunotherapy that has the potential to decrease COVID-19 progression, ER visits, hospitalisations, and mortality. More trials are going on and may provide a conclusion with high certainty. Practical guidelines were suggested for optimal uses of some NMAB such as Bamlanivimab and Etesevimab.
Because monotherapy of Etesevimab was found to be less effective and may be associated with worse clinical outcomes in hospitalized COVID-19 patients it should be used only in combination with etesevimab. These drugs were associated with the potential for serious hypersensitivity reaction, including anaphylaxis and clinical worsening after administration, especially in critically ill COVID-19 patients. The high cost and limited supply represented a major limitation for their use in counties with low income.
Several studies have been published on the encouraging outcomes obtained with convalescent plasma therapy in the treatment of COVID-19, the most notable characteristic being the lack of side effects. The use of COVID-19 convalescent plasma to treat critically sick patients has been approved by the FDA. Several requirements were provided to ensure the favourable outcome of this approach. A protocol for the production of anti-SARSCoV-2 sera was suggested.
The main issue is getting a sufficient number of donors with a COVID-19 history. An early meta-analysis came to the following conclusion: Convalescent plasma treatment appears to be safe for COVID-19 patients, with significant decreases in serum viral loads and most patients becoming virus-free following transfusion.
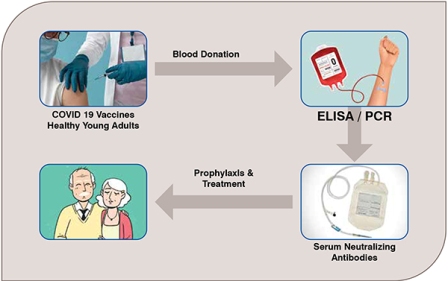
In adults <35 years a high antibody levels, against SARS-COV-2, was documented in the serum samples after 2nd dose of two authorised COVID-19 vaccines, the efficacy and safety of authorised COVID-19 vaccines was assured in several systemic reviews and meta-analysis.
Taking blood samples from young adults who have received two doses of COVID-19 vaccines is likely easier than taking blood samples from recovered patients. Authorised incentives could be implemented. Techniques are available for analysis of COVID-19 neutralising antibodies in serum.
Sera of COVID-19 vaccinated young adults likely represent a logical approach to be investigated for prophylaxis and early treatment of special population as kidney transplant patients against COVID-19. The risk/benefit should be assessed through well designed clinical trials.
References:
1. Roser, M., et al. Coronavirus pandemic (COVID-19). Our world in data 2021 [cited 2021 29 June]; Available from: https://ourworldindata.org/coronavirus.
2. Singh, S.P., et al., Microstructure, pathophysiology, and potential therapeutics of COVID-19: A comprehensive review. J Med Virol, 2021. 93(1): p. 275-299.
3. Pascarella, G., et al., COVID-19 diagnosis and management: a comprehensive review. J Intern Med, 2020. 288(2): p. 192-206.
4. Kang, Y. and S. Xu, Comprehensive overview of COVID-19 based on current evidence. Dermatol Ther, 2020. 33(5): p. e13525.
5. Ciotti, M., et al., COVID-19 Outbreak: An Overview. Chemotherapy, 2019. 64(5-6): p. 215-223.
6. Chakraborty, I. and P. Maity, COVID-19 outbreak: Migration, effects on society, global environment and prevention. Science of the Total Environment, 2020. 728: p. 138882.
7. Yang, X., et al., Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centre, retrospective, observational study. The Lancet Respiratory Medicine, 2020. 8(5): p. 475-481.
8. Benotmane, I., et al., Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int, 2021. 99(6): p. 1487-1489.
9. Benotmane, I., et al., Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int, 2021. 99(6): p. 1498-1500.