Although vaccine evaluation differs in various aspects from that for therapeutic drugs, many procedures specific for therapeutic drugs have been applied to vaccine evaluation.
In the recent years, many vaccine developments have focussed on therapeutic vaccines. However, prophylactic vaccines for infectious diseases continue to be of major importance. The infectious diseases can impact the public health and the socioeconomy. People are not aware of the threat of traditional diseases such as whooping cough (pertussis) or diphtheria because of the high vaccination coverage. Japan experienced a recurrence of whooping cough when acceptance of Diphtheria (D) Tetanus (T) Pertussis (P) combined vaccine (DTP) lowered after temporary suspension of the vaccination due to two post-vaccination mortality cases in mid-1970s. Pertussis incidence and related mortality increased from around 300 and 0 to over 13,000 and 40, respectively, by 1979.
How do vaccinations protect us against diseases? A vaccine like Live Oral Polio works in the same way as natural immunity would work once a patient is infected. Many vaccines are given parenterally (intra-muscularly or subcutaneously) to induce mainly humoral IgG and, in some cases, cellular immune responses. Infection in many cases begins with the bacteria or viruses attaching themselves to the mucosal surface of the respiratory tract, digestive tract or genitalium to multiply to further transfer or secrete virulence factors into the blood stream to affect target organs. Mechanisms of pathogenicity and protection of vaccination would differ for different pathogens. The causative agent of whooping cough is Bordetella pertussis, which produces various virulence factors or toxins, but the etiology of the disease and protection mechanism of vaccination have not been fully understood. Pertussis vaccine is also given parenterally and is reported to induce humoral IgG and cellular immune responses. But the vaccine thus administered is generally not adequate for induction of secretory IgA which is crucial for the local immunity to prevent attachment of the bacteria onto the surface of the respiratory tract. Therefore, the current vaccination might not be preventing pertussis infection but would be protecting vaccinees by the cellular and humoral immunities from symptomatic disease. This means that the bacteria may not have been eliminated but likely to be multiplying, instead, on the surface of the respiratory tract of the vaccinated individuals and may be circulating in the population infecting those who are not vaccinated or with waned immunity. Therefore, it is important to keep the population sufficiently immunised for controlling the disease.
Vaccines should be definitely efficacious so that vaccinees could be protected. After implementing an effective vaccine, the number of incidents should decrease to the level at which a meaningful clinical efficacy trial is not feasible. This means evaluating efficacy based only on clinical study and developing a new product and modifying production process of existing products would become impossible. It is essential, therefore, to develop a reliable laboratory model for efficacy evaluation. Such model should be able to evaluate the vaccine quality to induce functional protective immunity. A model for evaluating immunogenicity of vaccine antigens to induce antibodies that can bind to antigen would not always be adequate without ensuring protective function of the induced immunity based on a reliable laboratory protection model. Some of the well known models are the potency test for pertussis vaccine in mice and that for tetanus vaccine in guinea pigs.
Severe adverse events
The current safety evaluation of vaccines is similar to that of therapeutic drugs being based on clinical evaluation in which a couple of lots are evaluated in subjects enrolled in a way to eliminate bias as far as possible. While therapeutic drugs target a limited number of patients, vaccines target the whole healthy population and should be definitely safe. Once a vaccine loses its public reliance on safety, people may avoid the vaccine which could lead them to more severe sufferings from the infection.
Some general confusion might exist in interpreting the results of clinical safety trials. It is easy to find the expression "vaccine with proven safety in a clinical trial" in formal documents or guidelines. But the important question is, can vaccine safety be really proven in a clinical trial? A clinical trial can only ensure applicability of a vaccine. Results of a clinical trial can not be used for denying causal relationship between adverse events and vaccination because, although not randomly selected, routine vaccination is for a much bigger number of subjects compared to that of a clinical trial and would be more likely to involve those who are highly sensitive to vaccine toxicity than in a clinical trial.
To discuss about the adverse events in a vaccination, we need to take the lot-to-lot variation and sensitivity variation of individuals to vaccine toxicities into consideration. It is generally not feasible to examine people's sensitivity to toxicity of vaccines due to ethical reasons. However, there could be an exceptional case that creates no ethical problem. In the late 1970s to early 1980s when developing or newly implementing acellular pertussis vaccines (aP), various clinical studies were done in Japan. And among them, there were many reports on febrile response to aP lots. Data on the rate of febrile response of over 37.5 degrees celsius of more than 100 infants each to aP lots in such reports were summarised. The probit-transformed response rate and endotoxin (A cell wall component of gram negative bacteria can induce fever to the recipient) content of stud y lots are shown in Figure 2.
There was a clear correlation between endotoxin content and probit of febrile response rate, but febrile response rate varied for the lots containing a homogeneous level of endotoxin. This variation in the response rate would reflect the variation in the rate of sensitive individuals in the vaccination groups for the lots. This fact may suggest as in Figure 3 that sensitivity of individuals to vaccine toxicity would distribute, in most cases, in a log-normal manner and that toxicity of vaccine lots would also vary. Therefore, lower the level of toxicity, fewer the sensitive individuals. There may be some individuals who are sensitive to the lot when its toxicity was the highest level among lots.
Even a lot with a higher level of toxicity would cause no adverse reactions so far as it is administered only to those who are not sensitive to higher levels of toxicity. But a lot with lower toxicity, if administered to a highly sensitive individual, may cause an adverse reaction. However, if toxicity was lowered to the level of having no sensitive individual, no adverse event would occur. Annually, two cases of mortality and three encephalitis cases on an average used to be reported among more than 5 million doses of whole cell pertussis vaccine (wP) in Japan before 1974 (Table 3). On the contrary, almost no such severe adverse events have been reported for aP (Table 4) whose measurable toxicities were reduced, at least, to 1 in 10 of the level of average wP, although the relevant toxicity or toxicities are not known. The relevant toxicity might be some unknown biological activity that was detoxified in parallel to the measurable toxicities and may behave differently to a future modification of production process.
WHO has been encouraging to investigate adverse events following immunisation (AEFI) cases as quickly as possible when reported to collect relevant information for analysis. It is generally considered to have a need case definition criteria for AEFI but there is no clear guidance yet. Severe AEFI is quite rare and the number of cases could be further reduced by reducing toxic activities of vaccine lots as shown in the previous section (Figure3). For a meaningful approach to AEFI analysis, it is necessary to have quantitative toxicity parameters to evaluate vaccine over individual lots as shown for endotoxin. Furthermore, as seen for febrile response and endotoxin, vaccine lots with similar endotoxin content varied in febrile response rate to show that occurrence of adverse events depends not only on the vaccine quality but also on the probability to administer to sensitive individuals. Therefore, a meaningful approach to AEFI analysis would be to identify such toxicity parameter by correlation analysis on probability of AEFI and the parameters as seen in the example of endotoxin and febrile response in Figure 3.
Local reaction to vaccine, if severe and frequent, may affect public reliance on safety. It is evaluated by the surface observation in a clinical study. This procedure is not appropriate for detecting injection site injury, if any, by a vaccine dose given deep in the muscle. Such clinical evaluation would not be sufficient to prove that a vaccine is safe enough to cause no severe tissue damage at injection site.
It is necessary to evaluate the safety at least in a histopathological examination. To facilitate meaningful interpretation of results, two different products such as a newly developed product and an exisiting one of the similar vaccine can be compared at least in more than three laboratory models, for example, by injecting subcutaneously to mouse footpad, intra-dermally to rabbit back and intramuscularly to mouse thigh. If a significant difference in tissue injuring effect was consistently detected between the products in comparison irrespective of the model, the products did differ in their tissue injuring effect (Figure 4). Can we then be sure that only response of human tissue would not reflect such difference?
As discussed here, public reliance on safety of vaccine is essential to maintaining high vaccine acceptance. All the blame of those related in not making necessary efforts can not be shifted to official guidelines for the sake of public health.
Efficient control of population immunity and disease
No one can be aware when and how endemics begin. Once an endemic is suspected, health officials are asked to take urgent action to prevent it. If the endemic is limited to a certain generation, information on age bracket immune state would help urgent investigation. Information on immune state, such as proportions of those having protective immunity and those being susceptible, of each generation would be useful for controlling population immune state and infection. D, T and P antibody levels of Japanese population of 0 to over 60 years of age are surveyed every 5 years, in which data on approximately 75 subjects each for every 5-year age group are collected nationwide and analysed. Probit-transformed inverse cumulative rate of antibody positivity for several cut-off antibody levels should be a straight linear for normally distributed antibody titers as seen in Figure 1-a for diphtheria and Figure 1-b for tetanus. The straight lines fitted to the probit-transformed positive rates for each of the 5-year age groups could be used for estimating mean antibody titer and proportions of below protective level for each age group as shown in Table 1 for diphtheria and Table 2 for tetanus. Such information is essential for immunisation-related urgent action at the time of an endemic. This could easily be obtained by a small scaled surveillance using the normal distribution model. All DTaPs from these manufacturers comply with uniform regulations on lower limit for potency and upper limit for antigen content and are, therefore rather homogeneous. Distribution of antibody titers of the population so far immunised with such homogeneous vaccine lots seemed to be log-normal, which is essential to survey according to the normal distribution model. In case of age-limited endemic, an urgent identification of those necessary to be immunised would become feasible based on such information.
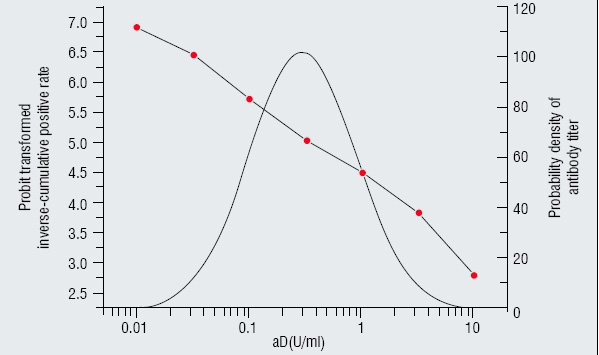
Figure 1a: Anti diphtheria
Table 1: aD antibody
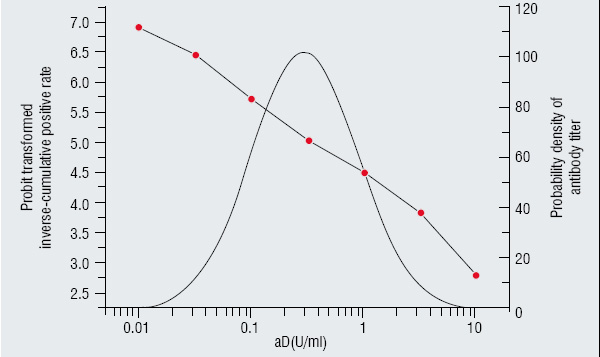
Figure 1b: Anti tetanus
Table 2: aT Antibody
* Limit level for protection
Table 3: Severe adverse events after DTP immunisation
Approximately two morality cases and three encephalopathy
(per Ca. 5 million doses) a year by wP during 1952 and 1974.
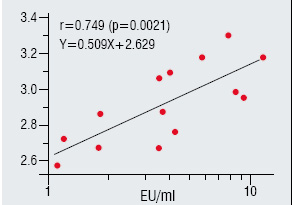
Figure 2: Rate of clinical febrile response to
endotoxin in practical vaccine lots
Endotoxin content of DTaP lots distributed log-normally. When endotoxin content increased, number of the susceptible and probability of showing febrile reaction increase.However, rates of febrile response even to the lots with equivalent endotoxin content significantly varied. This was because vaccinees of each lot were not randomly selected and probability of injecting to those sensitive to the level of endotoxin varied among the groups. Therefore, investigating lots showed a higher rate of adverse events (febrile response) would give no useful information. It is necessary, instead, to analyze the correlation of AEFI probability and a toxicity parameter as shown here.