Though Arab drug industry is increasingly contributing to the production of biotechnology products, particularly vaccines and herbal medicines, the main challenge is to achieve the regional objective of self-sufficiency in the production of essential drugs and vaccines.
In response to the 21st century challenges to the health sector, WHO established an important global commission on Macroeconomics and Health. One of the important conclusions of the commission report indicated that by the year 2015, the world could save up to 10.5 million lives every year by scaling up access to existing health interventions to prevent or treat infectious diseases, maternal and perinatal conditions, childhood diseases, and non-communicable diseases. Most of these interventions depend on the universal access to essential medicines and vaccines.
To achieve this objective, national and regional production of essential medicines and vaccines are important strategies that have been endorsed by ministries of health in the Arab countries.
The Arab world has 22 countries in the Middle East of which 19 are members of WHO Eastern Mediterranean Region. They have many important commonalities-they speak one language (Arabic), they are predominantly Muslim and share a common history. However, they differ greatly in socio-economic and demographic indicators. The population ranged from 0.725 to 72 million in 2005, and per capita GDP ranged from US$ 192 to 32,193 in 2004. Total health expenditure in Arab countries ranges from US$ 6 to 862 per capita, while medicines expenditure ranges from US$ 1 to 80 per capita.
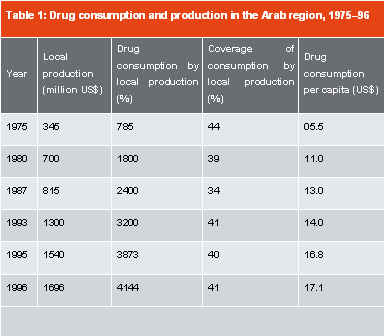
Data on the consumption and local production of medicines in the Arab countries are based on estimation from various studies. The total drug market rose from US$ 785 million in 1975 to US$ 4,144 million in 1996. However, the proportion of locally produced drugs in total consumption remained at just over 40 per cent (Table 1).
Data from the Union of Arab Manufacturers indicates that the Arab pharmaceutical market was worth US$ 6.20 billion accounting for 1.25 per cent of the global pharmaceutical market in the year 2003. In Arab countries, the local drug production constitutes various percentages of national drug market ranging from 0 per cent up to more than 90 per cent. In average, the local Arab drug industry covers 50 per cent of the total Arab drug market. It is estimated that the Arab drug market was worth US$ 10 billion in 2007. Saudi Arabia and Egypt are the two major markets in the region with a market size of more than US$ 1.2 billion each. The Egyptian market in some studies has been estimated to be around US$ 1.7 billion. Most of the locally produced products are formulations. In some countries, about 40 per cent of drugs are produced under license or in subsidiary plants of multinational companies. More than 90 per cent of the raw materials needed for local production are imported.
According to the World Health Organization report on World Medicines Situation, trends from 1985 to 1999, the value of total medicine produced has grown four times more than the world's income. Data in this report has also shown that only five industrialised countries produce almost two-third of the world medicines production.
It is important to note that the cost-effective local production of essential medicines can contribute to the universal access of essential medicines. Arab countries have, therefore, encouraged local production of medicines during the last four decades.
Arab pharmaceutical industry, which started in Egypt in 1930s, had passed through several phases that are characterised by state domination in the 1960s, followed by liberalisation in the 1970s and 1980s which attracted more private investment. The pharmaceutical industries nowadays are attracting more investments from the private sector in all the Arab countries.
The pharmaceutical industry in Middle East region recorded a combined annual growth rate of 10.6 per cent between 1998 and 2002. There are more than 245 pharmaceutical plants in the Arab states, and the number is increasing every year. They belong to the Reproductive Pharmaceutical Industries (RPI) and range from small-scale family enterprises to medium-scale public and private shareholding companies with diverse strategies.
Production is typically limited to the manufacture of patent-expired generic or branded generic medicinal chemicals and pharmaceutical preparations under license. Other main characteristics of the Arab drug industry include:
In all national immunisation programmes for prevention of serious communicable diseases particularly in children, the availability of good quality drug affordable vaccines represents one of the most efficient interventions. The sustainability of the continuous supply of the required vaccine represents a serious challenge to the national immunisation programmes in developing countries. New global changes and challenges include increased competition, difficulties in accessing cutting-edge technology, reduction in the number of vaccine suppliers, limited market and profit margins and decreased interest in vaccine production by the industrialised countries. They are putting additional strain on ensuring universal access and affordability of essential vaccines.
Data from 18 countries of the WHO / EMR region including 15 Arab countries show that in 2003, a total of 590 million doses of vaccines were purchased in the region, of which 12.5 per cent were from local producers.
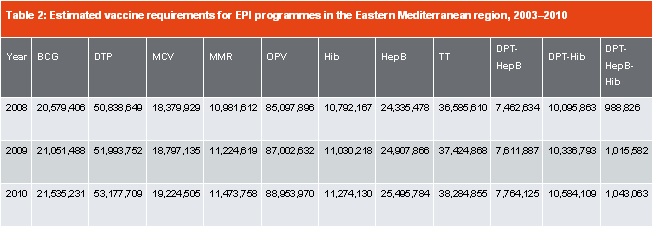
Table 2 shows the estimated needs of essential vaccines in the WHO / EMR countries. In Arab countries, only Egypt and Tunisia are vaccine producing countries. Table 3 shows production capabilities in Egypt and Tunisia.
Research and development activities in the Arab pharmaceutical industries focus on the manufacturing process and are often aimed at improving product quality. Small firms may not be involved in research and development activities. Recently, few companies particularly in Jordan and Egypt initiated some innovative research activities based on local medicinal plants.
Recently efforts are being made in Egypt, Saudi Arabia, Jordan and the UAE to start the production of biotechnology products, particularly vaccines and other biological products. The major areas that need to be addressed in order to develop research and development include lack of funding, lack of experienced human resources in certain specialised areas and lack of suitable environment to promote research and development. To address these challenges, the following strategies are suggested:

WHO / EMRO in collaboration with its member states including the 19 Arab countries developed the regional initiative on regional self-sufficiency in the production of essential vaccines and medicines.
The guiding principles for the development of this initiative are:
The regional vaccine self-sufficiency was discussed in 2004. It was agreed among the experts from the region that the regional vaccine self-sufficiency is a broad and inclusive concept. It means sustained and sufficient supply, of existing and new quality vaccines to meet present and future needs for priority diseases in the member states. The initiative also contributes to the establishment and strengthening of national vaccine production facilities and effective National Regulatory. Research, development and local production of vaccines, where feasible, needs to be strengthened to first meet the national requirements and then as a priority, the needs of other member states in the region. Effective and feasible regional and other cooperative mechanisms need to be established to foster such vision.
In conclusion, the challenges that Arab countries face in medicine production are:
Therefore, the following important strategies should be considered:
References
1. Macroeconomics and health: investing in health for economic development. Geneva, World Health Organization, 2001.
2. WHO, Eastern Mediterranean Region: Country Profile, 2007.
3. United Nations Development Programme (UNDP), Human Development Report 2007/2008, Fighting climate change: Human solidarity in a divided world.
4. WHO/EMRO, Regional Self-Sufficiency in Vaccine and Drug Production, EM/RC45/Tech Disc./1, August 1998.
5. Osama Kandil, "The Pharmaceutical Industry in the Arab World: Challenges, Controversies and Future Outlook" Drug Discovery Today, Vol. 9, No. 13 July 2004.
6. WHO, The World Medicines Situation, 2004.
7. WHO/EMRO, Vaccine development, accessibility and availability: towards self-sufficiency in the Eastern Mediterranean Region, EM/RC51/Tech. Disc. 2, September 2004.
8. WHO/EMRO, Regional Self-Sufficiency in Vaccine and Drug Production, EM/RC45/Tech Disc./1, August 1998.