Creating the right partnership is an essential skill in the complex eco-system of biopharmaceutical collaboration. This article outlines some approaches from safety sciences that allow effective collaborations to be designed not only to manage and mitigate risks, but to create the right interactions to develop sustainable high performance.
In today’s fast paced world of biopharmaceutical innovation, no one individual or single organisation can really work alone. Partnerships and collaborations are the normal way of doing business, whereas only a few years ago they may have been treated with suspicion.
The innovation cycle described in 2004 was a linear process. It was characterised by high cost, high attrition, long cycle times and a closed internal innovation loop. This allowed a close alignment of product and profit cycles. Today there are a number of different products, with classical NCEs, biopharmaceutical and gene/immune therapies brought to the marketplace by a range of investors, contract research and manufacturing organisations helping great ideas turn into useful medicinal products.
Experience shows that sustainable high performance of cross-organisational teams is not just dependent on having resources and a plan. To be effective, they need to be able to work together, share knowledge and integrate activities not only with themselves but also with a variety of governance and operating processes that may exist across organisational and cultural boundaries. With the development of digital technologies and artificial intelligence in support of decision making these complex systems will also increasingly need to integrate with automation.
Working in the interface between humans and machines, the science investigating the interplay between human cognitive processes, the environment and effective communication processes that relay the right meaning to operators have been developing for many years. This interface between humans and technical processes in engineering and systems design has led to the development of cognitive systems engineering or more broadly human factors.
Human Factor integration into technical work processes is an essential element of safety critical industries such as Aeronautics, Oil & Gas, Defence, and Nuclear Power. Minimising error in these industries is critical in preventing significant loss of life and/or environmental destruction as can be seen within the human history of accidents such as the Space Shuttle, Chernobyl and Oil Platforms in the Gulf of Mexico. Within the Pharmaceutical Industry, risks in application of powerful biological agents are also evident with the sad loss of life involved with the development of the CD28 antibody TGN1412 and FAAH modulator BIA 10-2474. These errors occurred across business-to-business (B2B)partnerships and have been well described with some key learnings being implemented across the industry.
Such accidents occur when there is alignment of a situation with an individual or team mistakes with an organisational or system failure that results in a loss. This perspective has been coined the ‘Swiss Cheese Model’. For a situation to result in a failure/loss it must align with gaps in a range of organisational, professional, regulatory, team, individual and technical defences as if it was aligning with the holes in the Swiss cheese. This approach was first outlined in the 1990s and has developed across many sectors including healthcare. Safety sciences recognise the crucial need to think about the system rather than the role of the individual to design the best system available. In the pharmaceutical industry, the plethora of individuals from patients, scientists, physicians, investors, and regulators who may be working in partnerships between private or public Companies, LLP and within the public sector all create potentially different views of the system.
In a first step toward supporting a Human Factor methodology, the UK Chartered Institute of Ergonomics and Human Factors Pharmaceutical Special Interest Group took a system engineering approach called STAMP (Systemictheoretic accident modelling process,) and reviewed top level development plans of a range of case histories to create a potential map to allow human factor integration into the medicines development system. The team developed four key emergent themes of relevance to all members of the system from a startup company to a government agency. These were:
Using these outputs was suggested to create a common and transparent language of all members of the innovation process.
Human factor or cognitive engineering approaches require us to take a system wide view of the interaction of individuals with not only automation, but other humans, managerial, organisational, cultural, and technical interfaces. Ideally, the system also brings in the end users and legislative and governmental aspects.
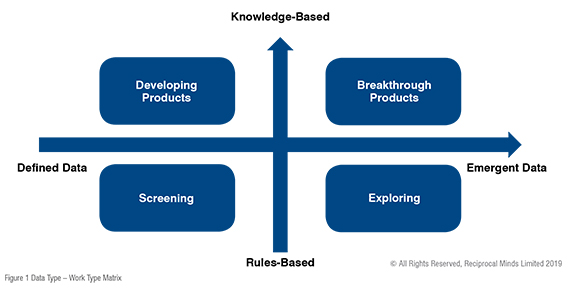
With medicine innovation, there is a dynamic and complex ecosystem that involves many contributors and stakeholders. We are only at the start of being able to describe the system as a whole. However, there are some approaches that could be implemented to enhance performance within the boundaries of business to business collaborations. The first step is to be able to describe the boundaries of the collaboration. One simple way involves comparing what type of data is produced (known at the start of the collaboration or emergent as the collaboration progresses) and what type of work is involved (Rule-based or Knowledge-based).
In the R&D eco-system, sustainable value (profits) can be created along the different phases in development by reaching key value inflection points. These are often pre-defined with known quality attributes for the project. For example, in the old linear model of innovation these were often defined by committing resources to start target validation, compound screening, optimisation of leads, committing to scale up and Phase I. However, as more data is created, the ‘asset’ trade-offs can be made between these attributes so that decision making becomes less binary (yes/no) and more dependent on the emergent data. This becomes more evident as the project moves through early and late clinical studies and through the more complex registration and post registration processes. As the project develops, the amount of information increases so that better decisions can be made around the key emergent properties of value, quality, safety and effectiveness.
Early models of cognitive systems engineering identified different types of decision making in working on the product (work agent). There are three types of work: skills-based, rules-based, and knowledge-based working/behaviours and problem-solving approaches. Skills-based work is often thought of as being automatic such as skilled motor coordination. This requires little conscious effort and is developed by practice. Rules-and knowledge-based working requires increasing cognitive demands. Rules-based working requires knowledge, but it is described in forms of processes and targets and runs the risk of becoming automatic and based on a set of artificial signs or data points where situational violations occur and can be managed. Knowledge-based working requires the highest cognitive demands. In this type of work, decisions are made not on the data (sign) itself, but on how the information is processed and may require the generation of new hypotheses and information. Exceptions and even dangerous violations in this setting can be managed. For example, an air crew being presented with contradictory data / information can form new perspectives to manage and resolve the situation. Examples such as the emergency landing of planes on the freeway and rivers are examples of this type of behaviour.
Within the context of biopharmaceutical drug discovery, it is tempting to think that all our collaborations involved knowledge-based workers. The high level of skills and knowledge required to operate laboratory services is not in question. However, with the complexity of the innovation ecosystem, work activities may be limited to rules-based activities (e.g., screening). Knowledge-based work around meeting multiple predetermined endpoints (e.g., review of phase 1 data). Less well-defined outcomes can even be emergent with decisions being based on the outcome itself (e.g., black box screens to explore biological pathways) or be required to work multiple conflicting data points to process (e.g., breakthrough innovations).
Within the context of today’s ecosystem, it may even be unlikely that organisations complete all these activities without any outsourcing or collaborating on work activities. Larger pharmaceutical companies often outsource rules-based work activities with preferred suppliers to capture the economies of scale that contract research organisations can provide. Smaller companies may work with a range of consultants to provide knowledge-based work activities such as understanding pre-clinical and/or clinical safety findings. In this case, a number of data sets may need to be processed to come to an overall conclusion and recommendation. These ‘service-based’ collaborations involve delivering a service from a vendor to a buyer, here a cognitive systems engineering approach would most likely provide a better opportunity to manage and mitigate errors and risks. When the system gets this wrong— as in Tegnero and Bial examples— the impact on patients and volunteers and their families is only too obvious. As we move into more complicated advanced technologies (e.g., gene modifications such as CAR-T immunotherapies), the impact of failure to manage errors effectively will not only be devastating to the patients and their families, it will also have significant impact on all the other members of the innovation system including researchers, institutions, and investors.
Risk-sharing collaborations can also be of a service type, where one partner offers services at discounted rates or in exchange for an equity state in the other. I am sure many start-up companies are really grateful for this type of a relationship. It brings the opportunity to grow. More complex risk-sharing arrangements are also common place. Here, smaller companies may be looking to scale their projects through the clinical development phases to reach a potentially global customer base. In this type of collaboration, the definition of what type of technical data is required may be less clear. The knowledge-based collaboration could be focussed on attaining registration / reimbursement whereas accessing a cellular screening assay might provide simpler rules-based (e.g. metabolites produced from cells) outcomes.
Figure 1 provides a summary of the types of collaboration that can be produced within the matrix. Of course, within the context of a fast-moving business relationship, it is possible that all aspects of the matrix may be involved. Knowledge of the type of collaboration allows alignment of communication processes and better understanding of the partners goals and motivations. This mutual recognition of what data is to be produced by what type of work activities allows design of appropriate processes to take into account human factors (e.g., rewards and motivations), cultural aspects, and governance processes. It also allows appropriate diligence on the skills and processors in prospective collaborators.
The psychology of human decisionmaking and judgement processes is complex. It involves the interplay of individual and group perceptions, cognitions and affective processes with regard to the technical aspects of the project. Cognitive systems engineering pays attention to these areas to create models that manage errors in safety critical activities so they can be mitigated effectively. This type of a systems thinking can, therefore, also be used to develop sustainable performance with collaborations. Risks of mis-communication and perception of data in the process of collaborative decision-making is often where mistakes and suboptimal performance can occur.
A simple tool to design appropriate decision-making processes can prove be essential. There are four key areas that include:
For all types collaborations, it is critical that there is a clear and consistent alignment on the knowledge and meaning that is taken from data. To do this effectively, explicit awareness of individual, organisational and cultural assumptions is needed on an ongoing basis, not just at the start of the collaboration. Project members may change in each partnering organisation and it is likely that business priorities and drivers will also evolve. With the long life-cycles involved in the biopharmaceutical industry, it is almost a guarantee that people involved at the start of collaboration will not be the ones involved at the end. For collaborations where the work creates a better understanding of the product, this is a particular challenge.
Organisational assumptions also need to be managed. For example, multinational organisations have a range of business processes that have often developed to manage risks associated with previous projects and operate in addition to any legislative procedures. Challenging the individual and organisational assumptions underlying data enables better decision-making.
Effective performance needs alignment of motivations and skills with an understanding of a sense of autonomy for individuals and partnering organisations. Agreeing up-front and reviewing regularly how decisions are made allows all members of the collaboration to develop a sense autonomy and alignment to the project. This process will also allow development of appropriate measures to be tracked. Three key areas appear to be crucial for the governance process. The first regards the technical production: ‘are we creating what we wanted to?’. The second is regarding the emergent properties of the collaboration: “are we providing value, quality, safety, and effectiveness for all parts of the innovation system?”The third and last area is on the engagement of staff: “are there enough resources for people to manage the demands of the collaboration?”Paying attention to these areas at a senior level will enable the creation of the human-centred organisation to maximise performance at all levels.
Conclusions
Taking time to adopt a cognitive systems engineering approach with B2B collaborations will enable all partners to develop the right processes and team members to maximise sustainable performance.