Millions of adults are diagnosed as obese each year. Many of these people are at risk of developing additional diseases, such as diabetes mellitus. The mechanisms by which obesity leads towards its complications are poorly understood. Recent research points to adipose tissue as the key target to treat obesity-related complications.
Millions of adults are diagnosed as obese each year worldwide. Many of these people suffer from a disorder known as metabolic syndrome, which includes symptoms such as hypertension and elevated blood cholesterol. They are also at risk of developing additional diseases, such as heart disease and diabetes mellitus. Obesity may, in fact, be a major cause of all these problems, but the mechanisms by which obesity leads towards its metabolic co-morbidities are generally poorly understood. Recent research points to adipose tissue as the key target to prevent and treat obesity-related complications. Obesity is characterized by excess body fat, which is predominantly stored in the adipose tissue.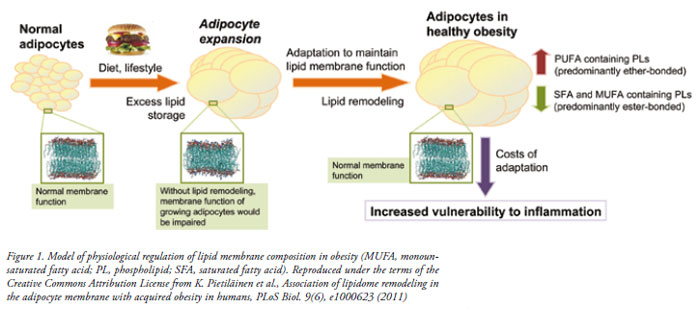 The specific mechanisms that may lead from obesity towards its metabolic complications such as insulin resistance and diabetes mellitus remain poorly understood. One attractive hypothesis, supported by the growing evidence from clinical as well as experimental model studies, is the “adipose tissue expandability” hypothesis. This hypothesis states that obesity associated metabolic complications are due to the limited capacity of adipose tissue to expand and therefore to store energy. If this limit of expansion is reached, the overflow of lipids leads to their deposition ectopically, leading to potentially toxic effects in peripheral tissues via the excessive accumulation of “lipotoxic” or pro-inflammatory lipid species such as ceramides.
The specific mechanisms that may lead from obesity towards its metabolic complications such as insulin resistance and diabetes mellitus remain poorly understood. One attractive hypothesis, supported by the growing evidence from clinical as well as experimental model studies, is the “adipose tissue expandability” hypothesis. This hypothesis states that obesity associated metabolic complications are due to the limited capacity of adipose tissue to expand and therefore to store energy. If this limit of expansion is reached, the overflow of lipids leads to their deposition ectopically, leading to potentially toxic effects in peripheral tissues via the excessive accumulation of “lipotoxic” or pro-inflammatory lipid species such as ceramides.
Individual’s capacity of adipose tissue may depend on genetic and environmental factors. While epidemiological studies suggest that there is a near linear relationship between the body weight and risk of diabetes mellitus as measured, for example, by a degree of insulin resistance, such an association may in fact be due to the “averaging effect” across a large population. Individually, adipose tissue expandability hypothesis would suggest that there is a threshold for body weight, as dependent on individual’s adipose tissue capacity. Reaching the threshold would then be accompanied by a notable decrease of insulin sensitivity. When averaging over a population level, increase of body weight would linearly associate with lower insulin sensitivity. However, in a population-wide analysis the information about the individual’s threshold is being lost.
This has important implication when considering early markers of risk of obesity-associated metabolic complications. While body weight is a well-established risk factor for diabetes, at an individual level it has very little predictive diagnostic value. Instead, biomarkers should be sensitive to the pathophysiological mechanisms leading to obesity-related complications. For example, marker sensitive to the status of adipose tissue may be able to detect when a person is close to reaching its capacity to store lipids in the adipose tissue and thus being at a higher risk of developing metabolic complications.
In addition to adipose tissue, liver is another key organ associated with diabetes risk. In fact, according to a recent study by The European Association for the Study of the Liver, based on current trends of incidence the non-alcoholic fatty liver disease (NAFLD) may affect 50 per cent of all US adults by 2030. NAFLD, which is characterised by the deposits of fat in the liver, mainly in the form of triglycerides, is a major risk factor leading to chronic liver disease and liver failure. In addition, liver fat is a major determinant of metabolic syndrome. There is currently no non-invasive test available for determining patient’s liver fat which is applicable in healthcare setting. Liver fat is usually determined by histology or estimated by magnetic resonance spectroscopy. The former is highly invasive as it requires the liver biopsy, so it is only applied in the case of chronic liver conditions. The latter may be too expensive for healthcare screening purposes. There is therefore a great clinical need for establishment of molecular markers sensitive to liver fat amount. Such markers could be applied for screening of patients, as well as in clinical trials aiming to treat the obesity-related complications.
 There is a need for paradigm shift from searching for early disease biomarkers in the epidemiological setting towards the search for molecular biomarkers of “intermediate phenotypes” which reflect the pathophysiological mechanisms behind the processes leading from obesity towards its complications such as diabetes mellitus. This will not only establish more powerful marker applicable in personalized healthcare setting, but also provide powerful tools for detecting persons at risk much earlier than currently possible.
There is a need for paradigm shift from searching for early disease biomarkers in the epidemiological setting towards the search for molecular biomarkers of “intermediate phenotypes” which reflect the pathophysiological mechanisms behind the processes leading from obesity towards its complications such as diabetes mellitus. This will not only establish more powerful marker applicable in personalized healthcare setting, but also provide powerful tools for detecting persons at risk much earlier than currently possible.
In the studies of adipose tissue, significant advances have recently been made by K. Pietiläinen et al. (K. Pietiläinen et al., Association of lipidome remodeling in the adipocyte membrane with acquired obesity in humans, PLoS Biol. 9(6), e1000623 (2011)). The team used mass spectrometry based lipidomics, a comprehensive strategy to profile molecular lipids, to study the fat tissue biopsies among several sets of monozygotic twins. In each twin pair, one twin was obese but metabolically compensated (i.e., “healthy obese”), while the other twin exhibited a normal weight. Because monozygotic twins share the same DNA and early upbringing, the impact of these factors on adult body mass phenotypes is accounted for, leaving other factors such as adult diet and lifestyle choices as the major variables.
When dietary intake was compared within twin sets, the obese twins were found to have lower amounts of polyunsaturated fatty acids in their diets than did their non-obese counterparts. Unexpectedly, the obese people had higher amounts of membrane lipids containing polyunsaturated fatty acids in their adipose tissues than did their non-obese twins. This finding is important because cell membranes are primarily composed of lipids, and different lipids can alter a membrane’s physical properties, such as fluidity and thickness. A novel approach was then introduced to model lipidomics data from membrane lipids by comprehensive molecular dynamics simulations. The computer modeling of lipid membranes indicated that the new lipids observed in the cells of the obese twins balanced each other in such a way that overall membrane fluidity was unaffected. The results therefore suggest that lipid-content changes in obese individuals might actually be an adaptation that serves to preserve membrane function as the cells expand. Additional analyses suggested that this adaptation can only go so far, and breaks down in the morbidly obese.
Furthermore, the elucidation of adaptive mechanism to maintain the membrane function in growing adipocytes also provides a clue about the potential novel targets to prevent or treat obesity related complications. In the same twin study, the statistical network analysis was applied to attempt to identify the regulatory mechanisms underpinning the adaptive changes and found the gene encoding the fatty acid elongase Elovl6 might be involved in fatty acid remodeling in obese people. This finding was further validated in vitro. When Elovl6 was silenced in an adipocyte cell line, the cells could no longer maintain the right level of the adaptive lipids observed in obese twins.
In summary, the study described above revealed how lipid membranes of adipocytes remodel to maintain normal membrane function in metabolically compensated individuals, and how this adaptation breaks down in individuals characterized by metabolic complications of obesity (Figure 1). Adipocyte membranes may therefore hold the answers about the early pathophysiological processes leading to diabetes. Consequently, measurement of adipose tissue membrane lipids or their correlates from serum could provide powerful early markers of diabetes risk, applicable in personalized healthcare setting.
The study may also help explain why obese people are at risk of developing inflammatory disorders such as diabetes mellitus: the kinds of lipids that accumulate in the adipocytes of obese people are precursors for compounds that are known to aggravate the immune system. Furthermore, the study suggests that the lipid network controlling the lipid membrane remodeling is amenable to genetic or therapeutic modulation. If small molecules can modulate this network to control for both membrane functional maintenance as well as for vulnerability to inflammation, new opportunities may arise for the prevention or treatment of obesity-related metabolic complications.
There is a great clinical need for establishment of early molecular markers sensitive to pathophysiological mechanisms leading to obesity-related co-morbidities such as diabetes mellitus. While epidemiological studies may be of help in search of specific risk factors associated with the disease, more studies are needed focusing on identification of disease-associated intermediate phenotypes and their markers. The latter may hold a better chance of becoming applicable in personalised healthcare setting because they detect presence of a specific pathophysiological process in each individual, which is indicative of disease progression. Furthermore, as discussed in this article, such intermediate phenotypes and their markers may also hold clues about novel therapeutic avenues