A Snapshot Molecular medicines have revolutionised the treatment and diagnosis of diseases. The advent of new genomic and proteomics based tools and techniques offer the opportunity to customise them at individual level. The application of omics science pose multiple challenges especially in developing countries. Nonetheless, the molecular medicine based diagnosis and treatment has tremendous potential to reshape the Indian market.
The patient and physician relationship involves a number of steps such as the advice on hygiene maintenance, chronic or non-chronic/infectious disease diagnosis, measures to limit the spread of disease, continuous requirement of diagnostic tools, techniques and diagnostic centres, pharmacy, disease monitoring, process and data management, obedience of statutory regulation and raising their concerns at the appropriate forums. From the patient’s point of view, the most important step of this relationship is diagnosis and treatment of the disease. On the other hand, primary healthcare physicians are always under pressure to provide the best diagnosis and treatment to patients in the absence of easily available, affordable and technically less complicated diagnostic methodologies and treatment options. Within last century, physicians and clinicians rely on their monographic education and symptomatic patient treatment. The transition of the century infused more molecular mechanism based medicines in the market and unfortunately in developing countries, these “Angles on Earth” are lagging behind in terms of their scientific knowledge and updates of the technological advancement.
Maintaining the patient’s health and enhancing longevity is the ultimate goal of all systematic and non-systematic efforts made by humans. Being at the apex level of evolutionary tree, they can easily modify the most affecting environmental and other factors. Survival pressure forced the human beings to switch their random approach to more disciplined and scientific studies of the surrounding flora and fauna for exploitation of the possible intervention tools. The systematic study of basic human biology is now evolved to decipher the concepts and phenomenon at molecular level as aptly summarised by Linus Pauling in 1956 that “Man is a collection of molecules”. The technological advancement in biological sciences leads to the unfolding of Human Genome and opens up the Pandora box against medical scientists to utilise the plethora of information for augmenting the level of human health and care through molecular diagnosis and sophisticated treatment with reduced cost. The application of Genomic and Proteomic information has huge R&D and market development potential along with the flourishing of a demanding, structured and more articulated service sector. The translation of these developments will revolutionise and redefine the patient-physician relationship.
The diagnosis and treatment approaches are continuously adjusted over the time in response to the endemics, epidemics and pandemics. The response to these adjustments against infections is well documented but non-infectious cases are perfected through symptomatic approach and partly supported via molecular approach. If the symptoms are overlapping for a disease, then it is still difficult for a physician to prescribe judiciously. Therefore, it is highly desirable that the modern medical science must be equipped with tools, which can pin-point the origin, type, nature, identification, possible remedies for a disease. The general requirement is the specificity of the tools and techniques desirable at the molecular level. The question is how to induct tailor made molecular medicines in the system and what are the problems and respective approaches required in doing so?
Traditional medicines have been used in several countries for thousands of years. In some Asian and African countries, 80 per cent of the population still depends on traditional medicine for primary healthcare [WHO (2008): Fact Sheet, No. 134]. In different cultures and regions, the traditional medicine system is in use without the parallel development of international standards and methods for evaluation.
The era of molecular medicine started in 1956 when ‘Linus Pauling’ published a paper on hemoglobin and showed that hemoglobin [HN3] of patients suffering from Sickle Cell Anemia had a different electrical charge than that from healthy individuals. Since then the molecular insight of various diseases has been explored resulting in the development of sophisticated and advanced physical and biochemical tools for the diagnosis and treatment of both infectious and non-infectious diseases. The continuous growth of the field establishes it as an independent discipline.
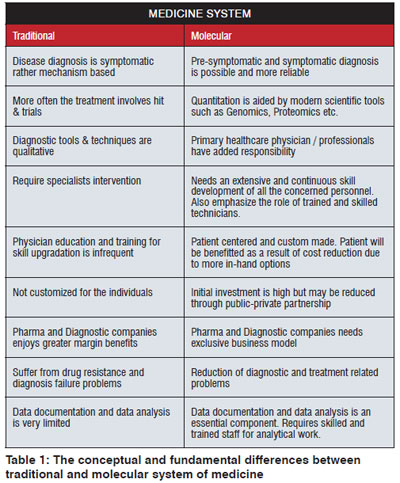
The current traditional system has several drawbacks especially considering the rationality of the diagnosis and treatment in comparison to the modern approach based on molecular profile. The lack of scientific justification of the practices, observation based and judgmental form of diagnosis renders the traditional medicine system susceptible to criticism. The popularity of the modern molecular medicines system is facilitated by multiple factors such as shortcomings of the traditional medicine system, scientific, technical and methodological advancements and quick healing (Table 1).
Molecular medicine is the outcome of conglomeration of physical, chemical, biological and medical techniques. This approach emphasises on cellular and molecular phenomena and their interventions. More scientifically, molecular medicines are application of genomic knowledge and stem cell sciences to human health with an ultimate promise of catching and eliminating diseases before the onset of symptoms. The current molecular medicine market includes the following segments: disease prevention, diagnostics, treatment, and patient care.
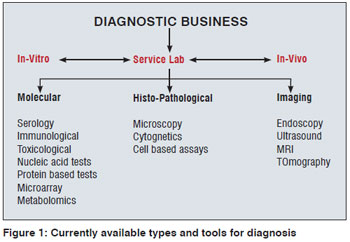
The current diagnostic business consists of three pillars: in-vitro diagnostics, in-vivo diagnostics and medical imaging. Presently, diagnosis is facilitated by service laboratories either in a public funded dispensary/hospital or private setup [Figure 1]. The basic molecular and histo-pathological tests constitute the bulk of the current diagnostic market segment in India. Medical imaging is not easily available as well as also not cost effective.
In-vitro diagnostics (IVD) involves the removal of tissue samples such as blood, saliva, biopsy samples from a living organism for examination in the laboratory, whereas in-vivo diagnostics involves observing and testing tissue and function in a living organism with the help of techniques such as medical imaging (x-rays, magnetic resonance, computed tomography etc.) and monitoring like electrocardiography, electroencephalography etc. With a compounded annual growth rate (CAGR) of 18.1 per cent from 2009 to 2016, in-vitro diagnostics has emerged as one of the most profitable markets for the Indian healthcare industry. The improving corporate hospital infrastructure and installation of automated and semi-automated biochemistry, immunology, and hematology equipment have enabled the market to achieve this healthy double-digit growth. The major reasons for the Indian in-vitro diagnostics market fragmentation are the low entry barriers, which led to the mushrooming of laboratories, and the absence of legislation leading to the complete lack of standardisation. Currently, only 150-200 laboratories in India have accreditation.
In-vivo diagnosis and medical imaging are slowly merging with each other. Asia-Pacific is likely to emerge as the fastest growing market with a CAGR of 17.5 Per cent. Medical imaging market consists of X-ray, Ultrasound, Computed Tomography, Positron Emission Tomography, Magnetic Resonance Imaging, Nuclear Medicine, Mammography and Fluoroscopy. Among all the imaging techniques, MRI & CT scan represents the largest segment (Source: PR web, San Jose, California, Nov.21, 2011). The global market for 3D Medical Imaging (software and workstations used in MRI and CT; and Ultrasound machines) is forecasted to reach US$5.9 billion by the year 2017, primarily driven by the technological advancements, resulting in improved imaging equipment. The medical imaging sector is a technology-intensive area, which has tremendous market potential as a result of advancements and integration of technologies. The advent of molecular biomarkers in future will greatly enhance the growth rate of medical imaging as well as the molecular medicine sector.
The medical biotechnology field is constantly flourishing world-wide and now acceptable to common man as a result of the value, safety and choice of products as well as availability of quality services with biotechnology advancements. Molecular medicine will contribute in improved risk assessment even in remote areas due to the availability of pre-packed kits and probably minimal of mechanical support. The rapid scientific progress in Genomic and Proteomics area will eventually lead to the high cost to return market benefits globally in molecular medicines sector.
India is a developing country and unfortunately 80 per cent of its population still has a daily income of Rs. 20/- only as per Tendulkar Committee report (The Times of India-'Reforms failed to bridge urban-rural divide', July 29, 2011). Furthermore, a newer technology is always expensive. Therefore, any discussion related to molecular medicines seems like making the hill out of a mole. Under such circumstances, the government funding agencies like Department of Biotechnology (DBT) and Indian Council of Medical Research (ICMR) has to play a greater role in making molecular medicines affordable through extending their support especially for ‘Omics’ based diagnostic tools and techniques.
Several Indian companies including Span Diagnostic Ltd. (Surat), Bhat Biotech (India) Ltd (Bangaluru) and Reamatrix India Pvt. Ltd. (Bangaluru) realised the true potential of this sector and are now driving the Indian market towards pre-packed diagnostic products. The market of laboratory diagnostics is growing and is said to achieve a growth rate of around 30-35 per cent in India. The initial investment will create market competition, which will boost easy availability, cost reduction and quality assurance down the line at the consumer level. Profits, Patent protected royalties and commercialisation opportunities will benefit the organisation that will spend money and will take initiative upfront.
Governmental support for the molecular medicine sector will not only help the R&D and manufacturing, but will also have a huge impact on clinics as a result of easy availability of human genetic maps, bio-marker assisted genetic analysis -- especially for chronic diseases like cancer and diabetes etc--, chip based detection and probably the availability of gene therapy. This kind of treatment approach needs mature understanding between a patient and physician and also patient’s willingness to embrace adventurous and miraculous therapy without hesitation.
The immediate challenge for medical scientists is to introduce patient-friendly methodologies and course of treatment driven by cutting-edge science, so that diseases can be diagnosed and treated accurately with minimum adverse drug reactions, drug over-dosage, drug resistance and accommodation of geographic variations. These challenges can be met only when the disease diagnosis will be based on accurate knowledge of molecular mechanism of diseases and tools to identify the peculiar mechanisms without overlapping. This is certainly a new approach for treatment and requires slow but definite transition from traditional to modern molecule based medicine system.
A futuristic and defined road map is required to achieve the ambitious goals associated with molecular medicine and diagnostics. There are a number of challenges, which need immediate and planned initiatives. These challenges are staggering at each level of development, to be carried out for establishing and capitalising on the potential offered by this area for the benefit patients. Financial support seems to be a foremost concern but there are other challenges including the availability of trained and skilled manpower such as technicians, physicians and even scientists for high-tech data interpretation, encouragement of entrepreneurship, assessment of associated risks, courage to invest, business models, basic infrastructure, technical education, establishment of technology transfer bridge between academia and industry, expertise in legal matters such as Patents, IPR etc., skilled manufacturing labour availability and above all the product designing capability. Marketing and making these products acceptable to general public at affordable cost, without compromising the quality is another major challenge, especially in a developing country like India.
The benefits of molecular medicines ought to be captured for health and wealth creation after thorough assessment of multiple factors as mentioned above. Education of the public and community at large forms another vital component of this process. Any change in the public attitude about the scientific progress comes slowly with increasing public familiarity, increased perception of benefits, withering of fear and associated dangers to the scientific innovations. New education programmes in interdisciplinary areas are required to generate skilled human resource and to create new intellectual property. Health professionals must be educated to ensure the rapid transfer of new knowledge and new information
There is an urgent need to make requisite public and private investment in genomics and related areas. Investment is required to create infrastructure, while private investment is needed for translating the existing and new knowledge into new opportunities. Open discussion of ethical concerns and strong regulatory framework will provide a major impetus to the development of this area.