Collaborations are a common business practice within the pharmaceutical and biotechnology industry. This article discusses both the strategic and tactical drivers for engaging in collaborations as well as some of the distinguishing features of the various types of collaborations used in this industry.
Collaborations are the voluntary, joint actions of two or more parties to achieve a common goal. This is a straightforward concept in principle, but often more complex in the real world.
Within the pharmaceutical and biotechnology sectors, one can easily identify at least six major classes of stakeholders, viz. private industry, academia, regulatory agencies, governments (policy and legislation), patient advocacy groups, and payers (public / private). Each of them can be involved in collaborative activities with one or more organisations in their own or other stakeholder categories. On a purely numeric basis, a single stakeholder could be involved in as many as 32 different kinds of collaborative interactions with organisations from their own or other stakeholder categories.
When discussing collaborations as a class of business transactions, it may be useful to consider why a corporation should engage in collaborations at all—after all, the typical outcome of any collaboration involves the sharing of the upside with an outside party. However, within that outcome lie the two fundamental reasons for engaging in collaborations. First, the belief that the apportioned, and anticipated return on investment from the shared outcome will exceed that which might be achieved independently. The second, and equally important driver for collaborations, is the recognition, by each party, that they have insufficient internal resources, e.g. cash, expertise, time, infrastructure or intellectual property, to independently achieve the desired outcome. Within the pharmaceutical and biotechnology sector, two fundamental objectives underlie much
of what we do: First is the need to get effective therapies and service to patients with unmet medical needs; and second is the necessity to create and maintain sustainable economic models which allow for the continuous creation of needed products and services. Collaborations are, in the broadest sense, tools which can be used to achieve these objectives.
In this context collaborations are classified into three categories—non-competitive, pre-competitive and competitive.
Non-competitive collaborations
The first group, non-competitive collaborations, is best exemplified by private industry-academia collaborations. Within the US, this model of industry-academia collaboration, with its concomitant flow of innovation from academia to industry has its basis in the Patent and Trademark Act Amendments of 1980 (University and Small Business Patent Procedures Act), more commonly known as the Bayh-Dole Act. Under the provisions of this act, universities, non-profit organisations and small businesses can obtain and retain ownership of, and patents on, inventions funded by the federal government. Additionally, the act requires that the universities actively engage in the commercialisation of these patented assets. With this legal foundation, American universities have, over the past 28 years, become increasingly important and currently essential sources of innovation for the pharmaceutical and biotechnology sector.
In non-competitive collaborations, such as those of industry and academia, the desired outcomes by each party are generally non-overlapping. For example, the industrial partner may be seeking novel product / service opportunities to develop within their own pipeline, or access to the specialised analytical or preclinical expertise of an academic laboratory. In a complementary fashion, the academic partner may be seeking to commercialise an asset developed within the university or to generate new scientific observations (publications) by evaluating the activity of a novel pharmaceutical agent in a preclinical model which is well established within the academic laboratory.
Although the qualitative distribution of outcomes, e.g. de-risked product / service opportunity, scientific publication(s), is relatively straightforward, the quantitative prediction of the value of these outcomes can sometimes be a critical point of disagreement / discussion / negotiation between the two parties. Such differences in valuation are to be expected. Each party is striving to maximise their return on investment (money, personnel and infrastructure), while simultaneously working to reasonably reduce / limit their costs.
Pre-competitive collaborations
The second major class of collaborations, pre-competitive collaborations, also have a legislative underpinning within the US, in this case, The National Cooperative Research Act of 1984 (NCRA), and the National Cooperative Research and Production Act of 1993. These laws were enacted to enhance the competitiveness of the US-based industries in an increasingly competitive international marketplace. The 1984 law clarified the application of anti-trust law to co-operative research ventures, and eliminated the treble damage awards associated with anti-trust violations. These benefits accrue to the members of a collaborative research consortium, provided that the consortium complies with the mandated disclosure of all participants and the purpose of the collaboration. The 1993 amendment extended similar provisions to joint production activities. Although more than 900 groups have registered under NCRA since 1984, registered collaborations in the pharmaceutical and biotechnology sectors represent substantially less than 5 per cent of the total.
To begin to probe why this collaborative model is apparently not favoured by the pharmaceutical and biotechnology sector, we can examine some of the main characteristics of this type of collaboration, and consider their match (or mismatch) with some essential aspects of the business model of the sector. The essential hallmark of pre-competitive collaborations is the focus on the development of tools and standards, and not the development of products and services. This aspect of pre-competitive collaborations is exemplified by one of the earliest and most often cited NCRA collaborations, Sematech. It was initially established as an industry / government collaboration for the development of advanced manufacturing methods for the US semiconductor manufacturing sector, and was created in direct response to the perceived domination of this manufacturing sector by Japan.
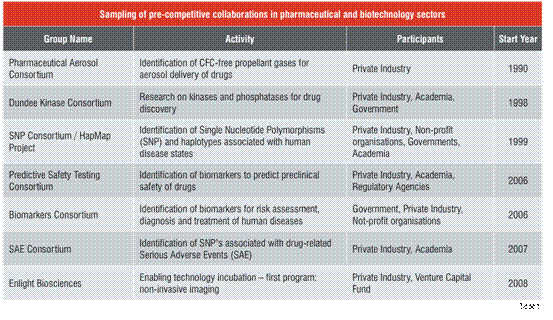
Table 1 is a sampling of pre-competitive collaborations in the pharmaceutical and biotechnology sectors. Several points are notable. First, as mentioned above, all of these collaborations are focussed on the development of information, tools and standards, putting them clearly in the pre-competitive space. Second, five of the seven are dedicated to generating massive data sets from various “omes”, genome, proteome, kinome etc. and analysing these data sets for relatively sparse information which, by itself, may have minimal competitive value. This general information, however when combined with proprietary and drug-specific information belonging to the individual participating companies, will be expected to provide a comparable, but unique competitive advantage to each participating corporation. The remaining two consortia, Aerosol and Enlight, are also focussed on problems whose solutions are likely to provide significant competitive advantages only when combined with additional proprietary, drug-specific information. This synergistic linkage of commonly held and proprietary information is a second defining criterion of pre-competitive collaborations.
To return to the possible reasons for the relative paucity of pre-competitive collaborations within the pharmaceutical and biotechnology sectors; although information / tools can be the subject of pre-competitive collaborations, they can also provide a competitive advantage to their owner. If an individual corporation has the resources and infrastructure to create a proprietary data set, extract useful information from that data set, and protect that information as trade secrets or patents, they will certainly do so. As noted earlier, a corporation is only going to collaborate in this effort when they have insufficient internal resources to complete the task on their own. Until recently, the complexity of problems, and size and complexity of the resultant data sets which were tackled by pharmaceutical or biotechnology companies were generally manageable as intramural projects, providing little impetus for pre-collaborative collaborations. However, with the arrival of “omes”, genome, transcriptome, proteome, metabolome, lipidome etc., some projects at this scale have become too massive and “drug-relevant” data too sparse to be cost-effective as intramural projects, and consequently, albeit slowly, are becoming the foci of pre-competitive collaborations.
Two additional factors may be contributing to the historically low numbers of pre-competitive collaborations. Until relatively recently, the standard pharmaceutical drug development model was inward focussed, with the belief that internal research and development could provide a sufficient number of commercially successful drug to sustain the growth of the corporation. Under that model, pre-competitive collaborations would have been unnecessary and perhaps unwanted. In recent years a more externally focussed drug development model has emerged, in which pharmaceutical companies have engaged in increasing numbers of non-competitive collaborations with academia. It remains to be seen if this trend towards utilisation of external resources will extend to the pre-collaborative space. An additional factor working against pre-competitive collaborations may be related to the extraordinarily long product development life cycle which is unique to the pharmaceutical and biotechnology sectors. Any strategic initiative in the pre-competitive space is unlikely to have a significant impact on a corporation until 8 to 10 years in the future. Given the shorter-term revenue and expense pressures which the pharmaceutical and biotechnology sectors currently face, the allocation of scarce resources to highly speculative, pre-discovery activities may not be viewed in a favourable light. A final factor in this matter may be simply one of cultural inertia. The sector as a whole has much more experience and familiarity with other kinds of transactions, e.g. mergers and acquisitions, product in-licensing and joint ventures. Even if pre-competitive collaborations offer some theoretical advantages, in the absence of clearly documented examples of strategic or financial benefit deriving from participation in pre-competitive collaborations, there may be a degree of reluctance to be an early adopter of this strategy.
Competitive collaborations
Collaborations between competitors are also regulated by large bodies of antitrust or competition law in all jurisdictions. Within the US, the Sherman Ant-Trust Act of 1890 briefly states, “Every contract, combination in the form of trust or otherwise, or conspiracy, in restraint of trade or commerce among the several States, or with foreign nations, is declared to be illegal”. Within this legal framework, the underlying need for competitive collaborations is the same as that of other collaborative types, i.e. the need to achieve an outcome which cannot be accomplished alone. To solve this need, a wide spectrum of collaborative activities between competitors (business-to-business transactions) has evolved.
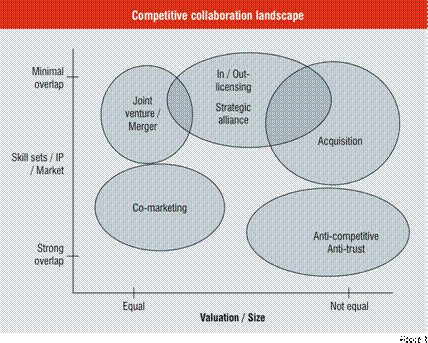
One way to structure and view the relationships between these different types of collaborations is to consider them in the context of a two-dimensional space as described in Figure 1. The first axis is the relative valuation and size of the two entities with respect to each other and the second axis is the degree of overlap of skill sets, or intellectual property or infrastructure between the participating entities. Utilising these axes, it is possible to place most competitive transactions within this space. For example, joint ventures and mergers are usually formed between entities of roughly comparable size and complementary skill sets or intellectual property. Licensing deals and strategic alliances are characterised by a minimal overlap in skills or IP (depending on the subject of the transaction) and are not particularly dependent on the relative size of the two parties. Co-marketing arrangements tend to be done between partners of roughly equal size, with a strong overlap in market, but some moderate degree of separation in call point or geographic coverage. Acquisitions are typically asymmetric transactions where the larger partner takes control of the smaller party and there is sufficient lack of overlap in market to avoid anti-trust / anti-competitive issues. Finally, the “buy your competition” business strategy, in which a large entity may wish to acquire a smaller party operating within the same market segment, may run afoul of anti-trust / competition laws. Refinements of this schema are clearly possible. Nevertheless, this approach, even in its current form, may provide some clarification or rationalisation of the type of collaborative transaction chosen to achieve a desired outcome.
In summary, collaborations involving the pharmaceutical and biotechnology sector, non-competitive, pre-competitive and competitive, may differ in their legal underpinnings, structure, complexity and value. However, all of these interactions are driven by: 1) the mutual recognition that some objectives can only be achieved by the combination of resources from two or more parties, and 2) the mutual expectation that the joint outcome will provide a positive benefit to each party that exceeds what might be achieved by proceeding independently.
Disclaimer: The opinions expressed in this article are personal opinions of the author and do not necessarily represent the opinions or positions of Genzyme Corporation or its senior management. Likewise, the identification of specific products, or organisations does not constitute an endorsement of those entities by Genzyme Corporation or its senior management. The commentary on, and summaries of, laws of the United States do not represent legal opinions on these laws.
*This article is based in part on a presentation, "Creating a Collaborative Pharma Model", given at the World Drug Discovery & Development Summit, Dec 2-4, 2008, Prague CZ.