Companies preparing for the implementation of pharmacoeconomic guidelines in Asia have an opportunity to use those resources for purposes other than justifying government reimbursement.
Many countries in the Asia Pacific region are both eagerly anticipating Korea’s implementation of pharmacoeconomic guidelines later this year, as well as remaining apprehensive. Governments are hoping that the reimbursement requirement will provide a framework that can be adopted to improve efficiency and contain pharmaceutical spending in Asian markets. Simultaneously, pharmaceutical companies are wary of the consequences.
Despite uncertainty as to where guidelines may appear next, many companies are taking a proactive approach and preparing for a future wave of pharmacoeconomic requirements in the region. They have started hiring health economists, looking at pharmacoeconomics models prepared by their global counterparts, and collecting local cost data to populate models. In addition, those that can demonstrate their drug’s “value-for-money” using pharmacoeconomics are voluntarily submitting data as part of their reimbursement application.
Generally, the pharmaceutical industry views pharmacoeconomic requirements from two different perspectives. On the one hand, they are preferred over continuous price cuts as a more predictable and transparent method for governments to increase the efficiency of pharmaceutical spending. The opportunity to demonstrate “value-for-money” is also seen as advantageous.
On the other hand, pharmacoeconomics represents a “fourth hurdle” that companies must overcome in the reimbursement process.
While these two perspectives have global applications, pharmacoeconomics presents a further problem for companies in low and middle-income markets. The main dilemma associated with presenting the cost-effectiveness of modern drugs in the region is that the costs of local resources are considerably lower than in high-income countries and drugs are not considered to be cost-effective unless they are cost saving.
As illustrated above, the cost-effectiveness or “value-for-money” of a drug is usually assessed in relation to its impact on government reimbursement decisions. However, opportunities to employ this economic technique extend beyond government reimbursement.
Patient level economic modelling offers an opportunity to demonstrate “value-formoney” at the patient level. The information derived can be used to educate both physicians and patients as well as demonstrate to a patient the value of prescribing a more expensive drug.
Interactive models can be designed so that the user can input patient characteristics and, based on modelled data extrapolated from clinical trials, project expected outcomes for different treatment options. Treating physicians can then use the model to demonstrate to the patient how treatment improves patient-specific outcomes such as the probability of relapse, hospital re-admission and return to work.
In publicly financed healthcare systems, such as Taiwan, prescribers are facing increasing pressure to contain costs.
In 2002, the Taiwan Bureau of National Health Insurance (BNHI) implemented global budgeting at the hospital level. Under Taiwan’s global budget system, hospitals receive lump sums from the BNHI to cover the cost of providing all medical services. While global budgets have successfully restrained the growth of expenditure, they have also limited the capacity of healthcare providers to embrace change in healthcare technology.
As a result, many hospitals are passing down budgetary pressure to the department and individual physician level. This practice has limited market access for new drugs since physicians, responsible for their own budgets, are reluctant to prescribe premium medications.
Pharma-driven patient-centred interactive models can serve to support physician decision-making in resource allocation as well as identifying patients for whom the drug represents “value-for-money.” For example, a physician could determine for each individual patient whether the premium product would provide a greater reduction in health outcomes, such as relapse or hospital re-admission, than the alternative treatment in consideration of the patient’s current health status. By prescribing a more expensive drug to those patients that the model identifies as having a greater risk reduction profile after treatment, the hospital can potentially reduce costs and reallocate those resources elsewhere.
Patient-centred models can be an important aid to help companies with premium products to break into markets where prescribers face increasing budgetary pressures.
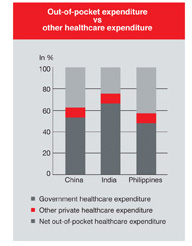
Several Asian countries provide minimal government financing in the area of healthcare. Consequently, the majority of patients in countries such as China, India and the Philippines must pay out-of-pocket for medical services and prescription drugs. According to the WHO, private healthcare expenditure in 2004 constituted 62.6 percent of total healthcare expenditure in China, 75.7 percent in India and 57.3 percent in the Philippines. Net out-of-pocket spending in China, India and the Philippines was 86.7 percent, 97.0 percent and 77.3 percent of total private healthcare expenditure, respectively.
For many individuals in these countries, healthcare presents a significant financial burden. The cost of premium drugs to treat an underlying condition may lead patients to discontinue treatment regimens if they are not well informed of the financial and health risks associated with forgoing treatment or non-compliance.
In these situations, an interactive model can serve to help patients understand how a treatment reduces health risks as well as demonstrating that discontinuation of therapy will prove to be more expensive in the long run. The impact on indirect costs can also be demonstrated to the patient. Indirect costs and benefits are often synonymous with productivity gains and losses. Productivity denotes the time patients or their families consume or free-up due to treatment and often is associated with work time. Pharmacoeconomic models designed for reimbursement applications often take a societal perspective and inclusion of productivity changes is contentious. By including productivity losses in a pharmacoeconomic model, an analyst assumes that a patient who is sick must forego employment during that period of illness. However, given a pool of unemployed labour, jobs may be filled by other members of the community and productivity changes may not be recognized at a societal level.
However, productivity is a patient relevant outcome. Demonstrating the potential change in productivity due to treatment translates into personal income for the individual patient and can be a strong incentive to reduce health risks by adhering to superior, albeit more costly, treatment regimens.
Companies starting to prepare for the potential wave of pharmacoeconomic requirements in Asia should embrace opportunities to utilise the technique in other areas of business, such as marketing. By developing patient-centred models, companies can demonstrate to patients in private pay markets both health and financial benefits of paying for more expensive treatments. Reducing the risk of productivity losses and more expensive hospitalization may
inform and incentivize patients to request premium drugs and maintain treatment regimens. In government-funded health systems, patient-centred modelling can be used to improve resource allocation decisions at the prescriber level and help companies break into markets that are facing increasing cost-containment pressure.
Patient-centred modelling and pharmacoeconomics can provide an innovative way to market drugs and penetrate barriers to access in both government-funded and private pay healthcare systems.