The continued rise in the number of biologics and advanced therapies in development has required the biopharmaceutical industry to adopt different strategies to de-risk these products compared to more traditional small molecules. This article will examine the technologies and platforms available and how they can best be applied.

Screening strategies for small molecules have evolved over decades to pan through thousands of molecules in silico and in high-throughput in vitro assays using the smallest possible amount of compound at minimal cost. As companies move increasingly to biologics and advanced therapies, the safety liabilities are changed, and the approaches and technologies required to de-risk the products change too. The applied technologies depend on several aspects including stage of development, availability and final formulation of test item, class of therapeutic, pharmacological target and mode of action. We discuss some of the more commonly used and useful screening technologies and explain how they work together to de-risk the development and safety of advanced therapies.
In silico tools to aid the safety screening of therapeutics have been available for a long time. The Derek system that identifies and has helped to catalogue structure-activity relationships was originally set up in the 1980s based on structural alerts for carcinogenic and noncarcinogenic potential (Ashby 1985). The system proved successful in predicting the mutagenic risk of small molecule drugs and has now expanded to consider the safety of predicted metabolites as well as parent molecules.
Whilst the Derek and related systems have proven invaluable to screen small molecules, the system is not applicable to biological therapeutics and advanced therapies. However, the success of Derek and other systems has led to widespread acceptance and adoption of in silico risk assessment approaches.
For gene therapies, the use of large and continuously evolving databases containing specific gene and protein sequences found in different species(e.g.BLAST, MegaBLAST and blastp)has proven indispensable for developing analytical methods, identifying potential off-target binding, detecting or verifying interspecies target specificities and transcript mapping across mammalian genomes.
In silico assessments are routinely used in the development of biological therapeutics to assess sites of possible post-translational modification and chemical stability. It is now possible to screen a library of over one-hundred billion fully human antibodies, computationally optimised in silico for therapeutic developability with complementaritydetermining regions sourced and incorporated at specific frequencies for maximum library diversity.
Use of these tools early in discovery helps to remove undesirable characteristics such as the risk of protein aggregation and immunogenicity from biological lead molecules. The approach also allows developers to conduct in silicoguided engineering of epitope sequences to reduce the immunogenicity of their preferred lead candidates.
Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) gene editing, and related gene editing techniques, have the potential to produce advanced therapies that radically alter disease progression and patient outcomes. This technology provides the possibility to edit out faulty genes and replace them with functioning copies of the normal gene.
Engineered CRISPR systems contain two components: a guide RNA and a CRISPR-associated endonuclease (Cas protein). The guide RNA is a short synthetic RNA composed of a sequence necessary for binding to the Cas protein and a modifiable spacer sequence of approximately 20 nucleotides that defines the genomic target to be modified. When designing a CRISPR Cas9 editing system the genomic target of the Cas protein can be changed by simply changing the target sequence present in the guide RNA, making it incredibly quick to engineer and widely applicable to a broad range of diseases. The system will edit where guided by the guide RNA, so if the guide RNA sequence is not sufficiently specific, or is inaccurate, it is possible that the system will edit the wrong gene, with potentially devastating consequences. In silico assessments of all potential hybridisation targets of any guide RNA are therefore critical in the design of guide RNAs to ensure that the correct gene is edited and that no off-target or unintended editing occurs. Establishing a rigorous and early validation protocol for new therapeutic candidates based on gene editing technologies is a prudent de-risking strategy.
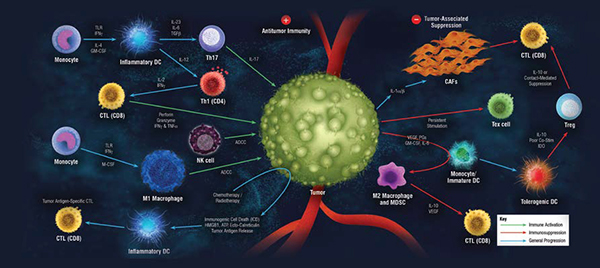
For nucleic acid-based therapeutics the combination of next generation sequencing (NGS) to sequence the whole genome and in silico evaluation is used to de-risk guide RNAs used for therapeutic gene editing tools, such as CRISPR-Cas9. As discussed above, in silico assessments help to predict and eliminate potential modifications leading to off-target effects, and it is NGS that is used to confirm that the editing proceeds as planned.
NGS is also applicable to target deconvolution and mechanism of action/mechanism of toxicity studies. When utilising NGS it is important to ensure that the data can be generated and processed in a meaningful way. This means that NGS is most powerful when coupled with a robust bioinformatics workflow and software, a pathways biology platform and deep expertise in data interpretation.
NGS is increasingly finding useful applications in proteomics. Researchers are using tools like CITE-Seq (cellular indexing of transcriptomes and epitopes by sequencing) and REAP-Seq (RNA expression and protein sequencing assay) among other techniques to understand mechanisms of disease by evaluating relationships between RNA transcripts and proteins in healthy and diseased cells, which will lead to the identification of specific therapeutic targets and eventually to innovation of highly targeted therapies.
Therapeutic mechanisms working through gene silencing or protein degradation, such as Proteolysis Targeting Chimera (PROTAC) and RNA interference (RNAi) biotechnology products, can lead to unintentional alterations in levels of endogenous proteins. In vitro off-target binding and activity assays appropriately used for small molecules are not suitable for these modalities. For this reason, new proteomic methods have been developed for the assessment of secondary pharmacology effects. One such proteomic platform has been developed using human cell lines to establish a prioritised panel of nearly 3000 proteins, which has been referred to as the “selected off-target proteome” as described in Liu et al., 2021.These researchers also demonstrated how they further refined the human proteome panel to produce a modifiable and expandable platform for identifying potentially adverse off-target or secondary pharmacology effects.
Use of RNAScope has become increasingly prevalent in development of novel gene therapies, allowing visualisation of transgene expression in situ. The technique utilises RNA in situ hybridisation (ISH) to allow the visualisation of single RNA molecules within individual cells. It is perhaps most commonly used with formalin fixed paraffin-embedded tissue from in vivo studies, but it can also be used to elucidate mechanisms of action and relevant biomarkers of safety in vitro. As an illustration, researchers have used RNAScope on non-adherent cells such as cytospin samples of patient-derived PBMCs (Chan et al. 2018), and to characterise neuronal cell culture models of chemotherapy-induced peripheral neuropathy (Eldridge et al 2021). The technique can also be used for visualising the expression of target(s) across multiple tissues even where there is limited availability of reliable antibodies. The use of RNAScope greatly enhances the ability to decisively determine transgene delivery and link localisation to the expected therapeutic benefits of gene therapy products using precision analytical tools.
Microarray technology, such as the Retrogenix cell microarray system enables users to quickly screen ligands including viruses and CAR-T cells for binding to thousands of proteins expressed on the surface of human cells. The proteins have relevant post-translational modifications and are naturally folded. Platforms are designed to include plasma membrane monomers, but also heterodimers. Secreted proteins have also been included by tethering to the matrix. The system can be run with either fixed or unfixed cells.
Thousands of full-length human plasma membrane and tethered secreted proteins are spotted onto microarray slides. Human cells grown over the top become reverse-transfected resulting in cell surface expression of each respective protein at distinct slide locations. The test molecule or cell is applied, and specific binding analysed and confirmed using an appropriate detection system. Detection methods include immunofluorescent labels/tags or radio label.
With broad coverage, such systems enable the detection of even low affinity interactions with a high degree of specificity and sensitivity. This information can be generated early in the design process to ensure target interaction, but critically target specificity, minimising the risk of off-target effects. As part of a CAR-x discovery program microarrays are used to screen the antigen recognition element initially and subsequently the whole engineered CAR-T or CAR-NK cell product to provide confirmation that binding to the target and off-target binding has not been inadvertently altered during the cell engineering and production process.
Cell-based systems lend themselves to higher throughput as compared to organ on a chip and are being more widely used for de-risking of advanced therapies.
Different virus serotypes and viral capsid variants are known to have different patterns of tissue tropism and antigenic properties. Chemical modification of capsids by adding receptor-binding moieties can enhance tropism. Also, by chemically masking the original receptor-binding moieties, the capsid can be shielded from neutralising antibodies that can inhibit effective distribution of gene therapies into their target tissues. Some of these approaches are described in Castle et al (Castle et al 2016). One of the earliest examples of modifying adeno-associated virus (AAV) tropism successfully by capsid engineering used the insertion of short peptides into the surface of the AAV capsid to confer affinity for a receptor expressed on the target cell (Yang et al 1998). Primary cells are regularly employed during this development process to screen new capsid constructs for improved tropism to the target cell. These in vitro screening studies ensure, not only that efficiency of transgene expression is increased to improve efficacy, but the safety risk of the therapy is reduced as the target cell population is more selectively targeted.
iPSC-derived cell lines have many similarities to normal human cells in terms of the way in which they respond to test items. By reprogramming the cells to become more differentiated and phenotypically neuronal or myocyte in characteristic, they become much better models to assess the individual cell type susceptibility to pharmacological action or toxic insult.
iPSCs from patients offer a further possible advantage as they hold the promise of producing neuronal or other cell lines with characteristics of patients with a wide range of diseases. These represent excellent models for screening the activity of many types of therapies. Patient iPSC-derived cell lines incorporate the weaknesses and susceptibilities of these patients own neuronal cells, making them excellent models to assess the comparative safety of new therapies in patients with particular diseases compared with healthy individuals.
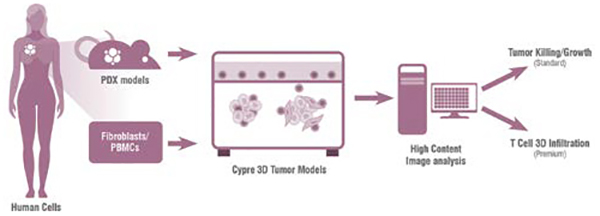
Artificial intelligence (AI) is now a powerful tool which can be applied to significantly improve the safety de-risking process early in discovery, with AI-driven pipelines of biotechs expanding at a very fast rate. Data from screening studies with DNA-encoded libraries together with high throughput in silico data are screened through AI-enabled computational platforms. These platforms leverage a wide range of in vitro and in vivo models and along with computational predictive models to help identify targets, predicting ‘druggable’ characteristics and target selectivity of molecules from a vast space. In terms of safety, AI can also be used to predict potential interactions and by leveraging publicly available data or proprietary databases can predict potential on- and off-target safety liabilities. A major advantage of AI systems is that they include an active learning loop, referred to as machine learning, which helps to improve the accuracy of prediction and to identify advanceable lead series or candidate molecules leading to a very high success rate, which improves as more data is gathered. Critically AI can also be used to screen billions of molecules virtually, reducing costs and resource requirements and improving the discovery process by more efficient use of molecular biology, public and private databases and other resources.
We have discussed some of the approaches that comprise an arsenal of powerful tools for de-risking novel biotherapeutics and advanced therapies. Often used in combination, the application of these tools in early development of advanced modalities can lead to even more effective, more selective and safer treatments for patients.
References