I’m originally from Belgium and I studied to be a chemical engineer with a specialisation in biochemistry and biotechnology. When I started out, it was my goal to get into the biochemistry or biotechnology industry as a plant director but when I graduated from university, my first job was teaching chemistry, biology, and math. Teaching was a very good experience because it helped me to understand leadership. I realised how important it was to influence, motivate, and empower the students. I learned that this type of leadership role was a good fit for me so I decided to change my career path.
I joined Analytical Instruments in 1999, as the European Sales Manager. At that time, it was part of IONICS*. I had several fantastic and inspirational mentors where I learned the ropes and started building the sales team. We had success in growing the business and I moved up the ranks. Analytical Instruments was acquired by GE and finally by SUEZ. I’ve been with the company for 21 years, so I must love it! The things that motivate me are still the same:making the world a cleaner place, environmental sustainability, and most importantly, developing our fantastic people.

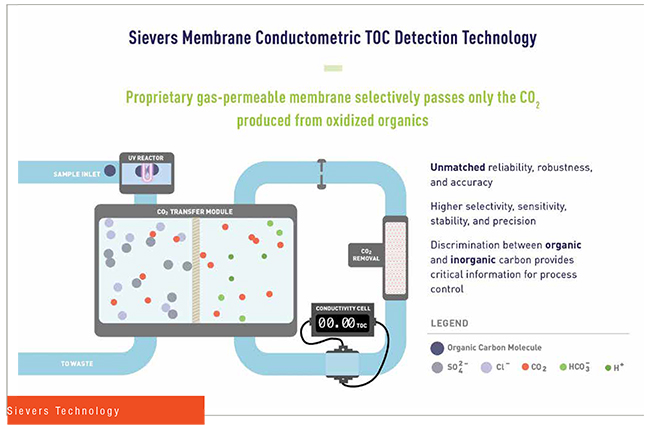
When I think about Sievers, I think about innovation and being at the forefront of technology to help our customers. We really listen to our customers and find creative technical solutions to solve their issues. This mindset comes from our heritage. Sievers has been a pioneer in the analytical instrumentation industry since introducing membrane conductometric Total Organic Carbon (TOC) detection technology to the market in 1993. Developing a better, faster and more efficient solution for our customers was our goal then, and it is still our goal today.
The newest product in the Sievers portfolio is the best example of what Sievers represents. Using our expertise in compendia water testing, we have launched groundbreaking technology for endotoxin detection.
Launching the Sievers Eclipse* Bacterial Endotoxins Testing (BET) platform was driven by finding a better, faster solution to endotoxin testing. We listened carefully to our customers about their challenges using the 96-well plate. We went back to the drawing board and developed a faster, simpler, and more environmentally sustainable solution. All of those things working together are the reason we launched Eclipse and we’re very excited about the future of endotoxin testing.
The global pharmaceutical industry has four fundamental parameters that are used to measure the chemical purity of water for production: conductivity, TOC, endotoxin, and bio-burden. Those parameters are used in a wide range of pharmaceutical applications and are highly regulated. TOC, conductivity, and endotoxin are metrics that manufacturers must monitor to comply with regulations, but compliance is only the beginning. They also allow you to go beyond compliance and achieve optimisation, process understanding, and process control. When using accurate and reliable technology, TOC, conductivity, and endotoxin data are valuable tools that can help improve cGMP processes and product quality.
The pharmaceutical industry has always searched for ways to improve product quality. Water quality became a focus since it is a key input for product quality. As a result, the analytical instrumentation industry focused on improving TOC analysis in the laboratory. The industry measures TOC on the parts per billion, or ppb level. When measuring a drop of water in an Olympic sized swimming pool, accuracy is critical. In the past, the analytical instrumentation industry focused on improving the accuracy, precision, and robustness of TOC analysers.
Over time as instrument accuracy and precision continued to improve, the industry began focusing more on efficiency. Lab analysis required a huge amount of grab samples. The analysts were sampling, waiting for results and then finally analysing the results to report back to quality teams so they can release water. The time and resources to do the work also increased as regulations became stricter. How can a pharmaceutical facility improve efficiency? One of the ways is by increasing automation.
One of the key trends in the industry is moving from laboratory monitoring to online monitoring to save time, money, and human resources. Sievers technology is used in both laboratory and online environments. This like-for-like technology makes moving from lab to online easier. The transfer of technology and methodology allows for better and faster implementation when making the transition.
Laboratory-based testing requires human resource time for sampling and analysis while online TOC analysers report data in real time. Online monitoring means plant supervisors don’t need to wait to get results so companies can move faster. The overall water monitoring process becomes more efficient.
Online water monitoring is becoming more and more important because the industry has realised it saves time and money, reduces errors, and improves product quality. Manufacturers can use the data to drive greater process understanding and process control. The benefits of automation, process understanding and process control are outlined in the FDA’s guidance document on Process Analytical Technology (PAT). Online monitoring fits well within the framework for PAT.
The next evolution of online water monitoring for pharmaceutical grade water systems is facilitating Real Time Release Testing (RTRT). It is important to highlight that RTRT is not simply about installing an online TOC analyser. RTRT means much more than having access to data. It is only when the data is used to release the water, when the real improvements in speed, cost and efficiency begin.
The validation of instruments is important for RTRT. Without proper validation, the value of real-time data is lost. One should consider the underlying TOC technology and the extent to which it can be validated against quantitative ICHQ2 (R1) guidelines. This level of validation can only be achieved with an analyser, compared to sensor technology. Without comprehensive validation, how can you trust the data generated from the instruments?
SUEZ offers Validation Support Packages (VSPs) for Sievers instruments that make validation easy. This gives you the assurance that the data can stand up to close scrutiny. To use real-time data and PAT to its full potential, technology must be qualified, and methods must be validated per regulatory requirements.
ASTM E2656 is an important guidance document outlining the aspects of RTRT implementation. RTRT is a powerful tool to improve efficiency, reduce costs, save time, and stay compliant to regulations, but the technology and implementation play a key role.
Historically, TOC data was measured in purified water systems and it continues to be a critical parameter. One of the growing trends is using TOC and conductivity in Cleaning Validation processes. Cleaning validation is not new to the industry. However, companies are evaluating ways to make their cleaning validation processes more efficient to reduce equipment downtime.
TOC and conductivity have become important quality metrics for determining cleanliness of cGMP equipment. They also offer several benefits compared to product-specific methods such as HPLC. TOC and conductivity give a more holistic view of cleanliness compared to other methods that only measure specific components.
TOC methods are also easier to use than HPLC, allowing more people to be trained, and trained faster. Anyone can be taught to operate a TOC analyser. The ease of use of TOC for cleaning validation allows organisations to manage human resources more efficiently, save time, and improve productivity.
Similarly to how the industry is advancing with water monitoring, the next trend in cleaning validation is moving from laboratory-based cleaning validation to Online Cleaning Validation. The FDA released its guidance document on PAT in 2004. Since then, we have seen the steady movement from lab to online water monitoring systems. We see the same trend in cleaning validation.
PAT lays the foundation for greater understanding and control for cleaning validation programs. Using TOC data enables further cleaning validation process automation and has become the best practice. Cutting-edge online monitoring technology is here today. The facilities that have not adopted TOC and conductivity for cleaning validation, and are not planning for the transition from lab to online, risk falling behind the competition.
The pharmaceutical industry is very demanding and forward-looking. We believe PAT is the future. PAT has been around for a while.It provides continuous process understanding, process control, and efficiency gains. Online analytical instrumentation technology and automation fit well with PAT and have clear, quantifiable benefits.
We at SUEZ focus on finding the most efficient solutions for our customers. Our vision is to focus on our customer’s needs and use our technology portfolio to help them. We started with improving TOC and conductivity instruments for the lab. Next, we added more support and services to those offerings to deliver more value. Most recently, we expanded our portfolio to next-generation bacterial endotoxin testing.
Now, it is our goal to partner with our customers to bring them to the next stage in their technology journey. That could be transitioning from lab to online water monitoring, RTRT, endotoxin, or online cleaning validation. SUEZ is focused on bringing technical expertise to our customers. Our customers can trust us to be a partner and to deliver results they can count on.
*Trademark of SUEZ; may be registered in one or more countries