Alliances can capitalise on the synergies between biotech’s innovations in drug discovery on the one hand and the pharma expertise in moving novel candidate drugs from the clinic to registration and product launch on the other.
In the past few years we have seen the announcement of many drug discovery alliances between biotechnology and major pharma companies. Pharma companies have started to outsource increasing parts of their drug discovery R&D to biotechs, signalling a trend in a changing industry. The alliances seek to capitalise on the synergies between biotech’s innovations in drug discovery on the one hand and the pharma expertise in moving novel candidate drugs from the clinic to registration and product launch on the other. The drug discovery alliances signed by Galapagos over the past two years is reviewed and the risk-sharing model that forms the basis of these pharma-biotech partnerships, from both the biotech and the pharma perspective is analysed here.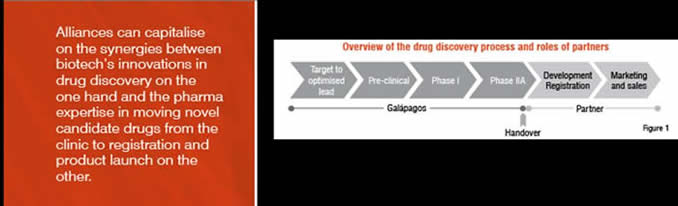
Rapid changes in the pharma industry
There is an overall consensus that the pharma industry is at a pivotal point in its evolution and is seeing the beginning of a lot of changes. The evolving patent landscape, with multiple patents on drugs launched in the 1990s expiring over the next few years, is one of the main drivers of these changes. Furthermore, the number of new medicines reaching the market is decreasing every year despite a rapid increase in the pharma industry’s investments in both R&D, and sales marketing over the past decade. The business model of creating and marketing blockbuster drugs is no longer sustainable as pharma companies have moved away from innovative new-mode-of-action-products to less risky me-too (molecules that act on a target for which drugs are already approved). This strategy has led to pipelines that lack innovative products and therefore will not be able to compensate for current medicines coming off patent. The current high-cost R&D pharma model needs to be replaced and pharmaceutical companies need to find ways to improve their R&D productivity, increase success rates, and cut R&D costs.
One of the ways in which the pharmaceutical industry seeks to address the challenges mentioned above, is by focussing on their core competencies: late stage development, registration, and sales and marketing of drugs. Furthermore, pharmaceutical companies are aiming to gain access to novel technologies or products and are looking for opportunities to expand their development pipelines through M&A, in-licensing of clinical drug candidates as well as through partnerships with biotechnology companies. It has become critical for pharmaceutical companies to establishing alliances with biotech patents to secure innovation to fill their product pipelines.
The biotech perspective
The evident strengths of biotech companies are the innovative power in early drug discovery, the spirit of entrepreneurship and the willingness to take early technological risks. An inherent weakness of many biotech companies, however, is the lack of sufficient funding to support their R&D efforts; the costs for drug discovery research continue to increase, and the access to capital is becoming increasingly difficult. Alliances with the pharmaceutical industry can serve to balance these costs and thereby effectively reduce the financial risks by sharing it with the partner. The essential element that defines the structure of such partnerships is the sharing of risks as well as rewards between the two companies.
With both pharma and biotech companies having incentives to pursue alliances, it should come as no surprise that deal-making is vigorous today. Over the last five years, more than 700 collaborative agreements were signed between pharma and biotech companies.
Risk sharing in drug discovery
The drug discovery process can be divided into several phases, starting from the search for drug targets through to launching and marketing a novel medicine. Figure 1 shows an overview of these phases including the main activities; each phase has its own profile in terms of requirements for technology and expertise, as well in terms of risk and funding needs.
Galapagos’ role in the alliances is to execute the early discovery and development phases of this process. This includes the discovery and validation of novel disease targets, conducting compound screening on these targets, identifying tractable chemical hits, pursuing hit-to-lead programmes, and developing resulting leads into candidate selection compounds through to a successful Proof of Concept in clinical research Phase IIa. At that stage the hand-over to the pharma partner takes place for all execution from Phase IIb onwards. The pharma partner has exclusive options to further develop and commercialise these compounds.
Overview of the Galapagos’ alliances
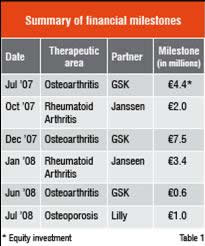
The agreements that Galapagos signed thus far provide the partner access to a number of Galapagos’ novel proprietary targets that play a role in a specific disease. In each alliance, Galapagos is responsible for developing candidate drugs and take these through to Phase IIa clinical Proof of Concept. At that point in the development, the partner gets the option to exclusively develop, manufacture and commercialise these candidates. In addition to receiving an upfront payment as technology access fee, Galapagos stands to receive discovery, development and regulatory milestones for each discovery program plus sales milestones and up to double-digit royalties on worldwide product sales. The revenues received from the alliance partners allow Galapagos to expand its drug discovery portfolio, from target validation to lead discovery, and to develop the resulting leads into candidate selection compounds. Since the first alliance was signed in June of 2006, Galapagos received €18.3 million in financial milestones; the main events are summarised in
Table 1. Galapagos drug discovery teams now handle over 30 novel targets in partnered R&D programmes.
Rationale for the alliances
Each party brought capabilities to the alliance that the other party valued; Galapagos was looking for a collaborator with deep pockets and vast experience in late stage clinical development, registration and commercialisation. The pharma partners needed a collaborator that would bring an innovative approach and expertise in early drug discovery.
The alliances have transformed Galapagos into a more mature drug discovery entity, through the expansion of Galapagos’ drug discovery activities to ‘pharma scale development’, and by reducing the portfolio risk while preserving long-term value of the pipeline.
Financial aspects
Through the alliance strategy described here, Galapagos is eligible to receive in excess of €1.7 billion in success-dependent milestone payments, up to double-digit royalties on sales of medicines that result from these programmes.
In structuring the deals, the alliance partners were able to define a creative funding structure that avoided the need for direct funding of Galapagos staff by the partners while securing milestone-based payments to cover Galapagos’ costs. The milestones in the alliances cover a wide variety of pre-agreed events, all the way from the identification of novel target sets through to regulatory approval of a new therapeutic. During the period that Galapagos is performing the drug discovery activities for the alliances, the milestone payments from the alliance partners compensate more or less the costs for Galapagos. That way, these large research programmes that in total have about 80 Galapagos researchers assigned, have a very limited impact on the cash burn of the company, assuming that the milestones are reached within the budgeted timeframe. After the handover of the product to the partner, further milestones and royalty payments will be pure profit for Galapagos as it no further incurs costs for that product.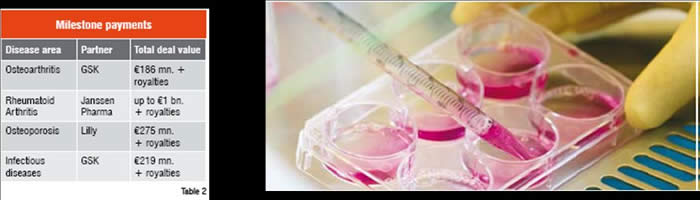
Advancing through partnerships
Risk-sharing alliances provide a novel way for biotechnology and pharmaceutical companies to capitalise on the complementary strengths of the partner, and to effectively de-risk the process of drug discovery. For the biotech partner the alliance provides the funding required to progress its product pipeline, and brings the late stage pharma development expertise and experience. For the major pharma partners the alliances secure access to novel technologies and options to product candidates that will help fill their product pipelines.
