The rise in counterfeit medicines worldwide, with concomitant problems of toxicity, instability and ineffectiveness, is often underestimated. It is a hidden risk because counterfeit medicines are largely overlooked in the official public health statistics.
The worldwide markets for pharmaceutical products are generally well regulated, efficient and profitable. It is their profitability that attracts many individual criminals and criminal organisations anxious to peddle counterfeit, illegally diverted and stolen pharmaceuticals.
Through the systematic collection, analysis and dissemination of information concerning the incidence of counterfeiting by the Pharmaceutical Security Institute (PSI) and its member companies, drug regulatory agencies, pharmaceutical manufacturers, international organisations and law enforcement officials are beginning to more clearly understand the extent of this problem.
Counterfeit Medicine: Is a medicine deliberately and fraudulently produced and / or mislabelled with respect to identify and / or source to make it appear to be a genuine product.
lllegal Diversion: This occurs when a genuine pharmaceutical product is approved and intended for sale in one market, but is illegally intercepted and sold in another market; these schemes are often accomplished through the use of false statements or declarations.
Pharmaceutical Theft: Is an illegal taking of medicines by robbery, burglary or theft; thefts are included as incidents when the value exceeds US$ 100,000.’
Incident: Is a discrete event triggered by the discovery of counterfeit, illegally diverted or stolen pharmaceuticals.
Documenting the problem
The Institute manages a unique database, the Counterfeiting Incident System (CIS), which is used to document incidents of counterfeiting, theft and diversion around the world. On a daily basis, incidents are identified by the careful work of a multi-lingual analytical team. Team members gather the facts from news accounts, police reports and drug regulatory authorities.
Incident reports from PSI member companies complete the collection activity. Comprehensive research produces a detailed picture of the individuals involved, their businesses, and their methods of operation as well as key identifying information.
The ‘2008 Situation Report’ identified 1,834 incidents as having occurred last year. This represented an increase of slightly less than 5 per cent over the total reported incidents in 2007.
Figure 1 illustrates the number of counterfeiting, illegal diversion and theft incidents recorded in CIS since 2002.
The following examples of incidents are provided to illustrate the types of incidents PSI documented during 2008. These prominent incidents illustrate the seriousness and potential danger involved with counterfeit pharmaceuticals.
The dimensions of counterfeiting are global
Though the incidents may vary from country to country, counterfeit pharmaceuticals are found in every region of the world. Over the past year, PSI has observed an increase in the number of countries affected by this problem.
In the ‘2008 Situation Report,’ incidents of counterfeiting, illegal diversion and theft were linked to 115 different countries. This was an increase of 3 per cent over the countries experiencing the problem last year.
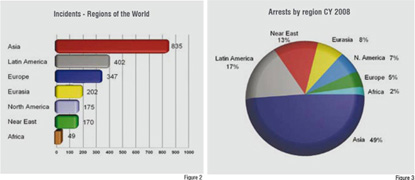
Figure 2 illustrates the geographic location and the corresponding number of incidents experienced in 2008.
Developing countries have been consistently linked to pharmaceutical crime incidents. In 2005, 76 per cent of incidents were linked to developing countries. An analysis of the 115 countries linked to incidents in 2008 reveals that this statistic has remained steady at seventy-six percent. This means that three out of every four incidents reported involved a developing country as a source, transit point or point of seizure / discovery of illegal pharmaceuticals.
The rise in counterfeit medicines worldwide, with concomitant problems of toxicity, instability and ineffectiveness, is often underestimated. It is a hidden risk because counterfeit medicines are largely overlooked in the official public health statistics. In cases where deleterious adverse reactions are not self-evident the problem often goes undetected. The patient is unknowingly taking ineffective medicines that fail to restore his / her health.
The following representative cases illustrate the potential harm to public health.
In June 2008, two young people in Singapore suffered hallucinations, anxiety and adverse reactions after taking the herbal supplement Relacore, which they bought over the Internet. According to the HSA, the product was marketed legitimately as an herbal slimming product, but contained a prescription slimming ingredient that exceeded the atic dose. The HSA was trying to establish if the patients bought a counterfeit version of Relacore.
In July 2008, the Jordan Food and Drug Administration (JFDA) issued a warning concerning the use of illegal sexual performance enhancers, cautioning that the drugs may contain toxic materials, such as yohimbine and strychnine, which could lead to death. The counterfeit medicines are sold in the form of pills for men and eye drops or gum for women.
In April 2008, Commissioner Andrew von Eschenbach told Congress that a blood-thinning drug was contaminated ‘by virtue of economic fraud.’ These statements followed a far-ranging investigation by FDA scientists. Up to 875 individuals were injured and 81 deaths may have been due to use of this contaminated drug. The adverse events included allergic or hypersensitivity-type reactions. In addition to the US, ten other countries were impacted.
According to the Health Sciences Authority, Singapore authorities seized more than 75,000 illegal products, including some sold as sexual-enhancement pills, after receiving reports of life-threatening reactions. The pills, manufactured in China, had more than five times the normal amount of active ingredient used to treat diabetes. The HSA confirmed that the total number of deaths associated with the use of these products in 2008 is ten.
 Following the death of a young woman in Seine St Denis, health authorities analysed the ‘Best life’ capsules taken by this woman. The results of the analyses showed the presence of Sibutramine, a drug substance contained in a prescription only medicinal product and requiring regular patient follow-up due to the risk of cardiovascular adverse effects. The ‘Best life’ capsules also contained phenolphthalein, a substance banned in medicinal products in France since 1999, and other plant derived substances that had laxative properties. Three arrests were made.
Following the death of a young woman in Seine St Denis, health authorities analysed the ‘Best life’ capsules taken by this woman. The results of the analyses showed the presence of Sibutramine, a drug substance contained in a prescription only medicinal product and requiring regular patient follow-up due to the risk of cardiovascular adverse effects. The ‘Best life’ capsules also contained phenolphthalein, a substance banned in medicinal products in France since 1999, and other plant derived substances that had laxative properties. Three arrests were made.
Dangers of the Internet
The Internet is an attractive environment for criminals since there are neither cyber police nor drug regulators on duty. Rogue internet pharmacies sell medications without prescriptions and supply counterfeit, stolen or unapproved products. They are based beyond the reach of the authorities as they carefully establish their operations in distant countries.
According to the WHO, medicines purchased over the Internet from sites that conceal their physical address are counterfeit in over 50 per cent of cases. Despite this fact, increasing numbers of countries have witnessed a significant increase of medicines ordered over the internet and shipped by mail. The latest figures show an increase in the number of pharmaceuticals being seized by customs agencies. Ease, discreetness, attractive prices and celerity are arguments used by internet drug seller to lure the unsuspecting into putting themselves in jeopardy.
Supporting investigations
While strategic analysis is important to policy makers, most often the investigator needs specific information to support an investigation. The Institute and its members help the authorities identify counterfeiters and their illegal operations. The following categories of information may be of value to investigators in initiating or focussing their inquiries.
During 2008, the Institute identified 766 new suspects living in 71 different countries.
PSI documented the arrest of 917 people involved in counterfeiting, illegal diversion and theft of pharmaceuticals. Asia and Latin America were the two regions recording the most arrests. See figure 3.
In 2008, 705 businesses were identified in relation to pharmaceutical counterfeiting incidents. The types of businesses recorded include pharmacies, distributors, websites, doctor’s offices and printing companies.
Training is a top priority
While sharing information is critical to successfully disrupting and dismantling criminal groups, it is particularly important to ensure that law enforcement officials are aware of the seriousness and pervasiveness of counterfeiting.
PSI and its members offer a variety of specialised training opportunities for law enforcement officers. Working with international organisations such as INTERPOL and the World Customs Organisation, PSI along with its members has provided training to representatives from more than forty different countries last year. Many of these representatives are critical to the anti-counterfeiting effort.
The pharmaceutical industry is strongly committed to assisting law enforcement and has set training as a top priority program for PSI.
Cooperation is the key to success
PSI is dedicated to working cooperatively with law enforcement agencies, drug control authorities and international organisations throughout the world to address the threat to public health and safety. Ultimately, only by working together and sharing information will these criminal organisations be successfully disrupted and dismantled.
All concerned government officials and business leaders are encouraged to: