Bio-summary tables are mandatory requirement for Clinical Summaries submitted to USFDA as a part of ANDA. These tables provide a standard format for data representation consistent with the FDA recommendations. This paper emphasizes the importance of Bio-summary tables which are reviewed for a new generic drug product by Division of Bioequivalence (DBE).
Bio-summary tables, also known as “Division of Bioequivalence (DBE Tables)”, are one of the main prerequisites for Module 2.7, submitted to the United States Food and Drug Administration (US FDA), as a part of the eCTD dossier. The FDA mandates the submission of these tables as PDF format and in MS Word document format in the appropriate eCTD/CTD locations (Module 2.7).

The main purpose of these summary tables is to provide a standard format for data to be in an Abbreviated New Drug Application (ANDA) in a concise format consistent with the current recommendations. The FDA provides specific instructions to fill in these tables. There are different types of summary tables based on the type of formulation and characteristics of the ANDA application.
The different types of summary tables for which individual instructions are provided by the FDA include the following:
The absence a of significant difference between test and reference with regards to rate and extent at which the drug is available at the site of action when administered at the same molar dose and under similar conditions is termed as Bioequivalence (BE). Hence, reports providing data from BE studies conducted to compare the rate and extent of drug absorption in vivo for a generic and corresponding reference product, are one of the critical components of ANDA submissions. The therapeutic equivalence of an active moiety as per the Regulatory standards depends on the determination of pharmaceutical equivalence along with establishing BE. A separate division [Division of Bioequivalence (DBE)] is designated in the Office of Generic Drugs (OGD), which is involved in the review of BE studies of new ANDA applications.
For ANDA BE submissions that contain the results of in vivo studies, the four major study report components are as follows: in vitro dissolution testing, bioanalytical methodology, clinical study reports and statistical analysis.
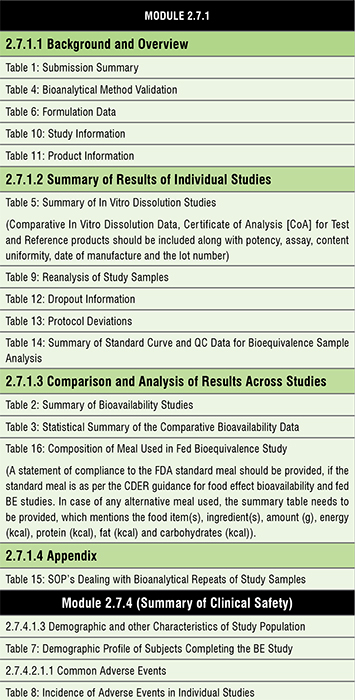
There are a total 16 BE summary tables for a typical product, which focus on the above information in a concise manner for DBE review. They are:
Table 1: Submission Summary
Table 2: Summary of Bioavailability Studies
Table 3: Statistical Summary of the Comparative Bioavailability Data
Table 4: Bioanalytical Method Validation
Table 5: Summary of In Vitro Dissolution Studies
Table 6: Formulation Data
Table 7: Demographic Profile of Subjects Completing the Bioequivalence Study
Table 8: Incidence of Adverse Events in Individual Studies
Table 9: Reanalysis of Study Samples
Table 10: Study Information
Table 11: Product Information
Table 12: Dropout Information
Table 13: Protocol Deviations
Table 14: Summary of Standard Curve and QC Data for Bioequivalence Sample Analysis
Table 15: SOP’s Dealing with Bioanalytical Repeats of Study Samples
Table 16: Composition of Meal Used in Fed Bioequivalence Study
Formatting points to be followed while filling the information in the above summary tables1:
Margins for the paper should be “1” for the top and bottom and “1.25” for the left and right sides
All text should be in Times New Roman, with font size 10
Default Table Style should be used while creating the tables in Microsoft® Word (Select Menu Table-Table Auto Format-Table Normal)
“Portrait” orientation should be followed for Table 1, Table 4, Table 7, Table 8 and Tables 10-16
“Landscape” orientation should be followed for Table 2, Table 3, Table 5, Table 6, Table 9.
As per the checklist provided by the FDA for an ANDA application, the above tables are to be placed in Module 2.7 in the following sequence.,
As per the RTR guidance from the FDA, it is mentioned that the FDA will RTR an ANDA, if the Study Information (Table 10) BE table is incomplete. The Study Information BE table compiles important information about study type and site locations and should be placed in Module 2.7 of the ANDA (along with the other BE summary tables).
The other minor deficiencies with respect to module 2.7 and summary tables that may trigger a RTR are as follows:
Conclusion
In summary, this information regarding the Bio-summary tables will help understand the standard format for data to be submitted to the Office of Generic Drugs in accordance with the current recommendations of the FDA for ANDAs. The pharmaceutical industry can take steps toward eliminating recurring problems related to summary tables by following the format and content of these tables and hence can submit the acceptable, complete, and well-organised BE submission to ANDAs without any RTRs.