Retention and recruitment of participants in clinical trials is often the most labor-intensive and challenging component. Poor recruitment and retention frequently pose a major barrier to the successful completion of clinical trials. This article examines the effective strategies for the research industry to improve the success rate in recruiting research participants using innovative recruitment methods.

It is widely recognised that clinical trial success is dependent on patient recruitment. Low enrolment rates have led to study times potentially increasing by up to two-thirds. Refusals to meet recruitment goals can have serious scientific, financial, and ethical implications. There are many intangible consequences that can be detrimental to investigators, sponsors, and participants. The most important aspect is that failure to recruit and achieve overall study goals can have a negative impact on patients. It hinders efforts to diagnose, treat or prevent disease. Despite multiple decades of efforts to identify and enroll study participants, recruitment continues to be one of the largest barriers to clinical trial success
Researchers have reviewed factors that could contribute to successful recruitment. They examined the design of trials, issues with study staff, recruitment strategies, and the need for revising recruitment targets and timelines. Other researchers have focused on increasing recruitment and retention by giving more consideration to participant contact, convenience, financial support for patients, incentives, compensation for participation, as well as other human factors. The most important phase of a clinical trial's life cycle, the upstream planning and design phases, may be the most influential for positively impacting downstream recruitment efforts.
Effective planning will require input not only from those who have traditionally led this effort but also from a range of stakeholders including patients and patient advocacy groups, sponsors, investors, site staff, and healthcare providers. Given the variety of factors that can affect recruitment for clinical trials, it is imperative to develop proactive and inclusive strategies that go beyond those that are specific to a particular study.
It will take more than simply reaching out to the community to find the solution. It is about understanding the complex motives and concerns of everyone. This requires the same level of individualised understanding that companies strive to attain, which taps into the personalities of their customers. Healthcare professionals have not yet considered factors such as these because they are not visible to them. These factors are only available through self-reporting techniques and focus groups. They are not reliable for uncovering the true motivations of people. To make progress, this issue must be addressed.
In today’s research landscape, recruitment is seen as an end-to-end process rather than an experience. The industry is missing an opportunity to engage potential participants through a sense of community and purpose. This is what a clinical trial should be built upon. This challenge was further highlighted by the experience of pharmaceutical companies during the pandemic. COVID-19 required the industry to test different methods of conducting trials, and a few companies tested the possibility of remotely running them. Initial expectations were that this would lead to increased participation and better recruitment. This has not been the reality.
This teaches us a simple lesson: People want human contact and emotional connection. It is crucial to make this connection, both because trials can be difficult and because participants can gain a great sense of purpose from their roles. People should never underestimate the power of feeling able to give back to others who are suffering from similar conditions. Maybe reducing friction inadvertently reduces the meaning of potential participants. These interactions can be harder to quantify emotionally, but they are just as important as monetary incentives. People are not rational beings that are only motivated by money. Trial recruitment should take a holistic approach to human motivations and be as focused on the sense of belonging and purpose that comes with being a participant. To help participants, find meaning in trials, this may require some friction and complexity.
Patients, as with all people, are predictably irrational. Although scientific and evidence-based understandings of human behaviour are crucial for driving sustainable behavioural change, it is not enough to know that people don't make decisions based only on rational information. Therefore, design and recruitment must consider both rational and emotional arguments. Even small changes can be difficult to implement, especially in large organisations that have been conducting trials in the same way for years. An outsider's perspective is crucial in this situation.
The industry must reevaluate its approach to trial design to address the urgent recruitment crisis. It must begin from the beginning, with how participants are understood. There is a business case to make that change. The industry can be helped by a facilitating new perspective.
Patients play an undisputed role in determining the care they receive. Since the days when patients were not included in the development of healthcare products was a controversial idea, we have made great strides. The healthcare ecosystem and the role of patients has been evolving in a promising direction in recent years. This has led to a deeper understanding of how the patient voice can and should impact healthcare.
The goal of including patients is now a top priority across the entire research landscape. This includes regulators, academia, and pharma. Patient partnerships have thrived because of the autonomy and confidence patients have acquired. Patients who have experienced the disease firsthand are an invaluable resource. They can clarify their priorities and needs well before we begin to discover and develop new therapies.
Many initiatives, including those of the Clinical Trial Transformation Initiative (CTTI), as well as many others, like the Patient Centered Outcomes Research Institute (PCORI), have given us valuable insights into patient engagement. These initiatives give us a glimpse into the types of metrics and operating models that work best, as well as the skills and experience that are most effective.
We have all come together in these cases and found common ground. We should not rest on our laurels. These experiences can be used to help us make progress in areas where we have only made modest or no progress in giving patients the active role that they deserve.
The United States is recognised as a world leader in clinical research. However, there are sub-optimal rates of participation in both industry and investigator led clinical trials in the USA. More than ever, the industry needs to develop recommendations for optimising recruitment, which are broadly translational and applicable at the site level.
There are some common strategies that research organisations can use to recruit participants, regardless of whether they are conducting a clinical trial or a community trial.
Preparing for Success
There are many factors about the study and your participant criteria that can affect:

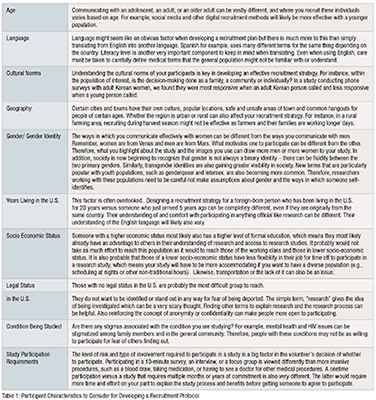
These factors will ultimately determine whether you will reach your recruitment goal. It is important to consider every one of these factors when planning your recruitment protocol and as you confront recruitment challenges during your study. A variety of participant and study factors can alter your recruitment strategies; therefore, these factors should be considered in the development of your recruitment protocol (see table 1)
The industry faces challenges implementing novel technologies to engage participants and collect clinical data. However, research suggest that by developing strategies to maximise participant and partner involvement and reduce participant and staff burden by simplifying participant experiences and staff workflows — Artificial Intelligence (AI), Machine Learning (ML), Natural Language Processing(NLP), Gamification, Wearables, Direct-to-Patient, remote direct to patients (RDCTs), and Agile (Hybrid) approaches should maximise recruitment, engagement and retention and disrupt the standard and outdated methods. It's hard to believe that patient recruitment is still a challenge despite all the advancements in clinical research. Traditional clinical research models are a challenge at multiple levels, which ultimately hampers the efficiency of clinical trials.
There are many barriers that could prevent patients from participating. These include:
Both at the site level and the sponsor, time and costs are often the greatest challenges in recruiting patients. The industry should be aware of the following aspects:
This might sound familiar to many in the industry. However, it is still amazed that these problems are not being addressed faster. To overcome these problems and increase patient recruitment, it is crucial to understand them fully.
Rebuilding trust with the public takes time. Strong campaigns and community engagement are necessary if we want to see industry improvements. There are also short-term solutions to the problems mentioned above. Patients and caregivers are the best place to begin. These insights can be used to inform patient recruitment strategies and study design.
References
1. Carlisle B, Kimmelman J, Ramsay T, MacKinnon N.Unsuccessful trial accrual and human subjects protections: an empirica analysis of recently closed trials. Clin Trials. 2015;12:77–83.
2. Krischer J, Cronholm PF, Burroughs C, McAlear C, Borchin R, Easley E, et al. Experience with direct-to patient recruitment for enrollment into a clinical trial in a rare disease: a web-based study. J Med Internet Res. 2017;19(2):e50.
3. Megan Molteni, “Meet the Company Trying to Democratize Clinical Trials With AI,” Wired, January 30, 2018, available at https://www.wired.com/story/meet-the-companytrying-to-democratize-clinical-trials-with-ai/.
4. https://www.klick.com/health/news/blog/semmedia/game-of-phones-digital-dominion-and-thedeath-of-tv/.
5. Harrer S, Shah P, Antony B, Hu J. Artificial intelligence for clinical trial design. Trends Pharmacol Sci. 2019;40:577–91 .
6. How to optimize patient recruitment -Achilleas Thoma, ForoughFarrokhyar, Leslie McKnight, Mohit Bhandari -Can J Surg. 2010 Jun; 53(3): 205–210 .
7. Mahon E., Roberts J., Furlong P., et al. Barriers to Clinical Trial Recruitment and Possible Solutions: A Stakeholder Survey. Applied Clinical Trials. 2015, September 3.