Currently, pharmaceutical industries are going under paradigm shift from traditional batch to novel continuous manufacturing. Such a novel continuous plant has been built at C-SOPS which is being adapted by several pharmaceutical companies. The Continuous Pharmaceutical Manufacturing (CPM) pilot-plant has been further modernised via utilising process system engineering methods and tools. Process model, automation system, real time monitoring, advanced Model Predictive Control (MPC) system, data management system and material tracing system are critical components of modernised CPM pilot-plant. A validated process model has been developed for in-silico studies and virtual experimentations. A process model plays a very important role to design, evaluate and tune the control system. The developed control system has been then implemented in to the continuous tablet manufacturing pilot-plant for practical demonstration.
Pharmaceutical industries as well as regulators (e.g. FDA) are strongly promoting smarter Continuous Manufacturing (CM). Few pharmaceutical products have been recently approved by U.S. Food and Drug Administration (FDA) to manufacture in continuous line and several others are on the way. There are several advantages but also different scientific challenges for this paradigm shift. Efficient and optimum process design, process automation, real time monitoring and control, material traceability, diversion of non-confirming products and real time release are few of those CM advantages which are scientifically challenging to address. Systematic Process System Engineering (PSE) methods and tools are,therefore, needed to efficiently overcome the obstacles on the path of CM shift and thereby to develop smarter continuous pharmaceutical manufacturing process.
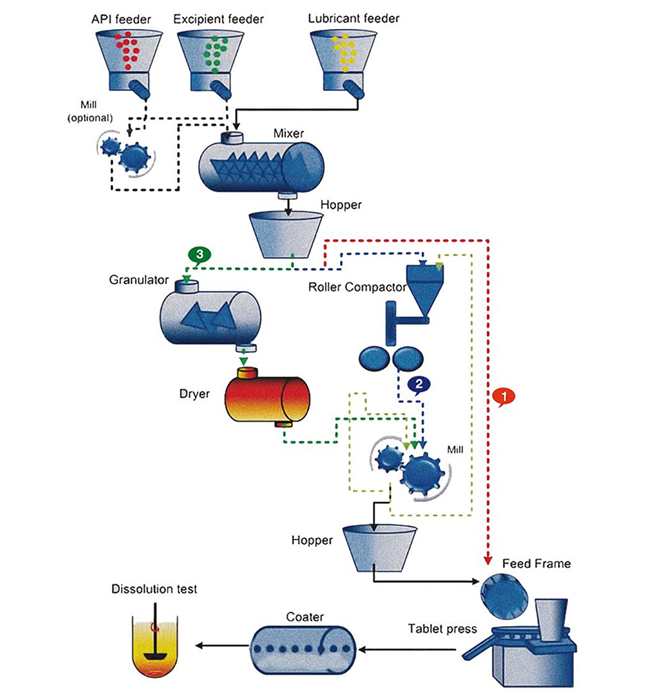
The objective of this work is to demonstrate the importance of system engineering to further modernise the continuous pharmaceutical tablet manufacturing process.
A flexible continuous pharmaceutical tablet manufacturing process is shown in Figure 1. As shown in the figure, there are three continuous pharmaceutical manufacturing routes: (1) via Direct Compaction (DC), via dry granulation/Roller Compaction (RC), (3) via Wet Granulation (WG). Some unit operations are common in all three manufacturing routes. The selection of DC, RC and WG routes depends on the type of formulation to be used to make the tablets. A continuous direct compaction tablet manufacturing process is a simplest route. Details of the process dynamics of the direct compaction line have been previously reported, and the open-loop as well as closed-loop operation has been extensively studied. The continuous manufacturing process via Roller Compaction (RC) is called dry granulation route. It is a preferred route for liquid sensitive formulation where wet granulation option is not feasible to use. The roller compactor converts the powder blend into ribbon. A mill is integrated with the roller compactor that breaks the ribbon and form granules. In the wet granulation route, the granules are first manufactured and then compacted to form tablets.
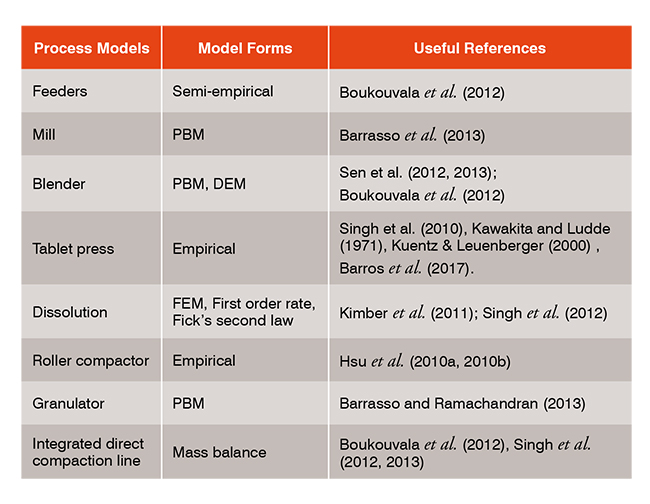
The model of each unit operation involved in continuous manufacturing process has been previously developed. The feeder model is reported in scientific literatures. A mill model is reported in Barrasso et al. (2013). The mathematical model for powder blending, an important but complex unit operation, has been previously developed as described in Sen et al. (2012, 2013). The model for the tablet compression process is previously reported in Singh et al. (2010). This model is based on the Kawakita powder compression model (Kawakita & Ludde, 1971)and Kuentz-Leuenberger tablet hardness model (Kuentz & Leuenberger, 2000). The dissolution model was adapted from Kimber et al. (2011). The model of roller compactor is adapted from Hsu et al. (2010a, 2010b). A population balance-based model for the granulation process has been presented in Barrasso and Ramachandran (2012). The PID controller model is available in the control section of the Process Model Library (PML) of simulation software gPROMS (PSE). The models for the different unit operations have been developed and included in gPROMS (Process Systems Enterprise, PSE) to facilitate the integrated flowsheet modelling. The control relevant process model involving transfer functions has been developed in Simulink (Math works) through step change response analysis (Singh et al., 2015; Barros et al., 2017).
The process model plays a very important role for design of an efficient control system prior to its implementation into the pilot-plant. It significantly minimizes the time and resources needed to implement the control system. The design of an efficient process control system is an interactive procedure that involves the identification of critical controlled variables, coupling of the controlled variables with suitable actuators (manipulated variables), selection of suitable monitoring tools, selection of control strategy followed by tuning of controller parameters, and model-based closed-loop performance assessment. A model library and a knowledge base are important supporting tools for control system design. An integrated mathematic model for continuous tablet manufacturing process that used for design of control system is a part of model library. The knowledge base consists the information about the process as well as the information about the monitoring and control system. The control system for continuous pharmaceutical tablet manufacturing process via direct compaction has been previously designed and evaluated. An engineering study on control of continuous pharmaceutical tablet manufacturing process via roller compaction has been also systematically performed. The control system for continuous wet granulation process has been designed and evaluated as well. Several studies have been performed on design and evaluation of control system around tablet press.
The automation of continuous pharmaceutical manufacturing process is needed to operate the plant through a centralised control platform, to collect the process operational data in real time and subsequently apply this data to control the plant in order to achieve desired product quality. Traditionally, the pharmaceutical product is manufactured through batch processing with very less or no automation involve and therefore this area of research need more attention. The continuous pharmaceutical manufacturing pilot-plant has been automated. Fieldbus, serial ports and OLE Process Control (OPC) have been used to integrate these unit operations with the automation system. All the data generated in these unit operations are collected and stored systematically in a data historian.
The continuous pharmaceutical manufacturing process need to be monitored in real time. The main objectives and associated challenges are as follows: (1) Identify and integrate different tools needed for real time sensing. (2) Identify inbuilt, external available and non-available sensors. (3) Demonstrate the application of existing sensors for real time monitoring. (4) Develop new methods for real time sensing if it’s not exist before. There are some spectroscopic and non-spectroscopic sensors and chemometric tools commercially available that can be adapted for monitoring of continuous pharmaceutical manufacturing process. However, these sensors, and tools need to be systematically integrated in order to successfully monitored the continuous pharmaceutical manufacturing process. The first step for real time monitoring is to build required calibration models. The calibration model is only needed for spectroscopic sensors. After building a good calibration model, the next step is to integrate it with a prediction engine and PAT data management tool for real-time monitoring and control of pharmaceutical manufacturing process. There are different tools commercially available that can facilitate it. The central idea is same in case of each tool.

It involves the integration of spectroscopic sensor (e.g., NIR) with the plant through chute. After that, the sensor outlet needs to be connected with a computer in which the sensor operating software is installed. Through the sensor operating software, the raw data (spectrum) are collected in real time. The raw data are accessed from a real-time prediction tool. The PLS model should be already uploaded in the prediction tool. The prediction tool employed the PLS model and uses the raw data as the inputs to the model and gives the signal of the variables that need to be monitored. The measured variable can then be sent to the control platform via OPC.
The process control system need to be implemented for real time product quality assurance. In order to implement the control loops into continuous tablet manufacturing pilot-plant, the first step is to create control loops (PID /or MPC) in control platform. Then tune the controllers. For MPC, a model need to be developed as well. The NIR signal is the input to this control loop that generates the actuator. Finally, the actuator is sent to the plant via a suitable communication protocol. The type of communication protocol depends on the unit operation where the control signal needs to be sent. For example, the control platform communicates with the feeder via field bus (e.g., Profibus/Device Net) and with the tablet press via OPC. Finally, the performance of the control system needs to be evaluated experimentally.
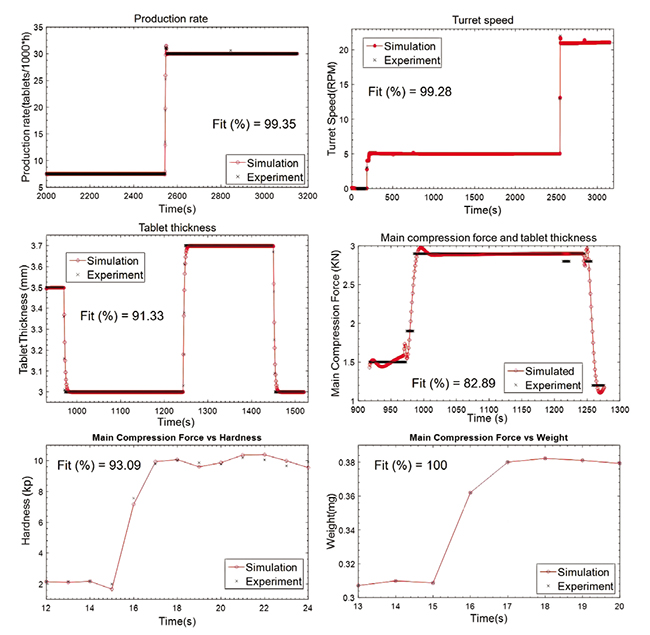
The control relevant process model has been developed. The model successfully can predict the actuator, critical process parameters and critical quality attributes. The tablet press unit operation has been considered here as a demonstrative example. The model performance for prediction of actuators, critical process parameters and critical quality attributes are shown in Figure 2. The results show the close agreement between model prediction and experimental results.
The process model has been successfully applied for design and evaluation of control system for continuous pharmaceutical tablet manufacturing process. Control architectures for 3 manufacturing routes (DC, RC, WG) of continuous pharmaceutical manufacturing have been previously developed. Feedback control strategy has been also compared with combined feed forward / feedback control strategy. Compared PID, MPC and hybrid control algorithms. Evaluated control system performance for set point tracking and disturbance rejection ability. Integrated gPROMS (PSE) simulation tool with MATLAB. Integrated gPROMS (PSE) simulation tool with DeltaV.
Feeder has been integrated with control platform via profibus connection. Co-mill and Blender has been integrated with control platform via serial ports. Tablet press has been integrated via OPC. Spectroscopic sensors have been integrated with control platform via different software tools. Non spectroscopic sensors have been integrated with control platform via serial ports.
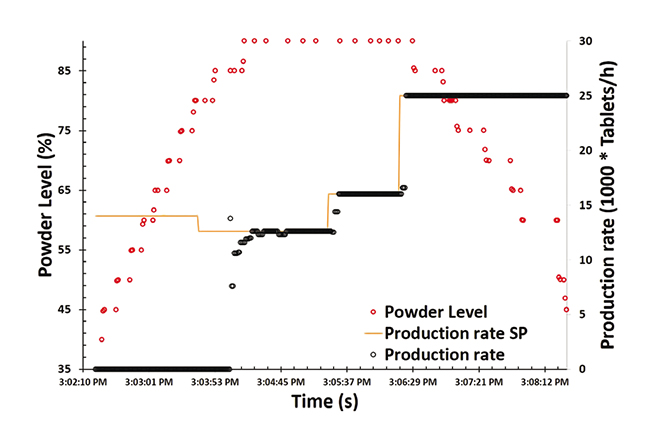
There are some inbuilt sensors in each unit operation of continuous pharmaceutical manufacturing process to monitor different variables in real time. Feeder weight, powder flow rate and feeder screw rotational speed have been monitored at feeding operation using inbuilt sensor. Impeller speed has been monitored at co-milling operation. Blender RPM has been monitored at blending operation. Tablet thickness, compression forces, ejection force, and operating parameters (e.g. fill depth, feed frame speed, turret speed) are monitored at the tableting operation using inbuilt sensor. Integrated supervisory sensors with plant and software tools for real time monitoring. Powder blend composition, uniformity (RSD) are monitored at the blending operation using NIR. Commercial sensors were not available for some variables which were desired to monitored in real time. Developed novel sensing methods for real time monitoring of powder bulk density. Developed method for real time monitoring of tablet weight. Developed method for real time monitoring of powder level in transfer pipe. The real time monitoring of powder level is shown in Figure 3. As shown in the figure, the production rate has been changed in order to change the powder level in transfer pipe. The sensor was able to monitor the powder level in real time.
Implemented supervisory control system into direct compaction continuous tablet manufacturing pilot-plant and compared PID with advanced Model Predictive Controller (MPC). Feeder-blender control to assure drug concentration has been experimentally demonstrated (Singh et al., 2014). Develop and implemented control system into connecting transfer pipes. It is difficult to run the continuous pharmaceutical tablet manufacturing process at steady state for longer time without the use of this controller. This controller also makes sure a consistent powder level all the time in front of PAT sensors. Implemented advanced control system into tablet press unit operation. Developed control system for pre-compression force (i.e. control of tablet weight via pre-compression force). Simultaneous control of pre and main compression forces (2x2 MPC) have been also demonstrated (i.e. control of tablet weight and hardness via pre and main compression forces respectively). Supervisory control of tablet weight (Cascade MPC) has been added as well.
Process System Engineering (PSE) methods and tools have been developed and applied to continuous pharmaceutical manufacturing process. The control relevant process model helps to design, tune and evaluate the control system prior to its implementation into the pilot-plant. CM pilot-plant has been automated to optimise the resources and to establish consistency in plant operation and data management. Real time monitoring and control system has been implemented into CM pilot-plant. The real time control of continuous pharmaceutical manufacturing is a significant advancement in pharmaceutical industry and is considered essential for real time release as well as patient safety.
Acknowledgements: This work is supported by the US Food and Drug Administration (FDA), through grant 5U01FD005535, and National Science Foundation Engineering Research Center on Structured Organic Particulate Systems, through Grant NSF-ECC 0540855.
References:
(01). Yu, L. Continuous Manufacturing Has a Strong Impact on Drug Quality. U.S. Food and Drug Administration (FDA). 2016, https://blogs.fda.gov/fdavoice/index.php/2016/04/continuous-manufacturing-has-a-strong-impact-on-drug-quality/. Last accessed (12.18.2017).
(02). Lee, S. Current FDA Perspective for Continuous Manufacturing. MIT-CMAC 2nd International Symposium on Continuous Manufacturing of Pharmaceuticals, September 26-27, 2016. https://iscmp2016.mit.edu/sites/default/files/documents/FDA MIT-CMAC for CM 2016 Ver6.pdf. Last accessed (12.18.2017).
(03). Pharmaceutical Technology Editors. FDA Approves Tablet Production on Janssen Continuous Manufacturing Line.2016,http://www.pharmtech.com/fda-approves-tablet-production-janssen-continuous-manufacturing-line. Last accessed (12.18.2017).
(04). Yirka, B. Eli Lilly develops continuous manufacturing process for chemotherapy drug. Medical press.2017, https://medicalxpress.com/news/2017-06-eli-lilly-chemotherapy-drug.html. Last accessed (12.18.2017).
(05). Singh, R. Model-based control system design and evaluation for continuous tablet manufacturing processes (via direct compaction, via roller compaction, via wet granulation). Book title: Process Systems Engineering for Pharmaceutical Manufacturing: From Product Design to Enterprise-Wide Decision-making. Publisher: Elsevier,2018, Volume 41 (1st Edition).
(06). Boukouvala, F.; Niotis, V.; Ramachandran, R.; Muzzio, F.; Ierapetritou, M. An integrated approach for dynamic flowsheet modeling and sensitivity analysis of a continuous tablet manufacturing process: an integrated approach. Comput. Chem. Eng.2012, 42, 30-47.
(07). Portillo, P.M; Vanarase, A.; Ingram, A.; Seville, J.K; Ierapetritou, M.G; Muzzio, F.J. Investigation of the effect of impeller rotation rate, powder flow rate, and cohesion on powder flow behavior in a continuous blender using PEPT. Chem. Eng. Sci.2010, 65, 5658–5668.
(08). Singh, R., Ierapetritou, M., Ramachandran, R. An engineering study on the enhanced control and operation of continuous manufacturing of pharmaceutical tablets via roller compaction. International Journal of Pharmaceutics2012, 438 (1-2), 307-326.
(09). Singh, R.; Ierapetritou, M.; Ramachandran, R. System-wide hybrid model predictive control of a continuous pharmaceutical tablet manufacturing process via direct compaction. European Journal of Pharmaceutics and Biopharmaceutics2013, 85(3), Part B, 1164-1182.
(10). Singh, R.; Sahay, A.; Karry, K. M.; Muzzio, F.; Ierapetritou, M.; Ramachandran, R. Implementation of a hybrid MPC-PID control strategy using PAT tools into a direct compaction continuous pharmaceutical tablet manufacturing pilot-plant. International Journal of Pharmaceutics 2014a, 473, 38–54.
(11). Singh, R.; Sahay, A.; Muzzio, F.; Ierapetritou, M.; Ramachandran, R. Systematic framework for onsite design and implementation of the control system in continuous tablet manufacturing process. Computers & Chemical Engineering Journal2014b, 66, 186-200.
(12). Vanarase A, Muzzio FJ. Effect of operating conditions and design parameters in a continuous powder mixer. Powder Technol.2011; 208: 26–36.(
(13). Singh, R.; Barrasso, D.; Chaudhury, A.; Sen, M.; Ierapetritou, M.; Ramachandran, R.Closed-Loop Feedback Control of a Continuous Pharmaceutical Tablet Manufacturing Process via Wet Granulation. Journal of Pharmaceutical Innovation2014c, 9, 16-37.
(14). Barrasso D., Walia S., Ramachandran R. Multi-component population balance modeling of continuous granulation processes: a parametric study and comparison with experimental trends. Powder Technol. 2013; 241: 85–97.
(15). Sen, M.; Singh, R.; Vanarase, A.; John, J.; Ramachandran, R. Multi-dimensional population balance modeling and experimental validation of continuous powder mixing processes. Chem. Eng. Sci.2012, 80, 349-360.
(16). Sen, M.; Dubey, A.; Singh, R.; Ramachandran, R. Mathematical Development and Comparison of a Hybrid PBM-DEM description of a Continuous Powder Mixing Process. Journal of Powder Technology2013, http://dx.doi.org/10.1155/2013/843784.
(17). Singh R.; Gernaey, K.V; Gani, R. ICAS-PAT: A Software for Design, Analysis & Validation of PAT Systems. Comput. Chem. Eng.2010, 34(7), 1108-1136.
(18). Kawakita, K.; and Ludde, K. H. Some considerations on powder compression equations. Powder Technol.1971, 4, 61–68.
(19). Kuentz, M, & Leuenberger, H. A new model for the hardness of a compacted particle system, applied to tablets of pharmaceutical polymers. Powder Technol. 2000, 111, 143–145.
(20). Kimber, J.A., Kazarian, S.G, Stepánek, F. Microstructure-based mathematical modelling and spectroscopic imaging of tablet dissolution. Computers and Chemical Engineering2011, 35, 1328–1339.
(21). Hsu, S.; Reklaitis, G.V; Venkatasubramanian, V. Modeling and control of roller compaction for pharmaceutical manufacturing. Part I: Process dynamics and control framework. J. Pharm. Innov.2010a,5, 14-23.
(22). Hsu, S.; Reklaitis, G.V; Venkatasubramanian, V. Modeling and control of roller compaction for pharmaceutical manufacturing. Part II: Control and system design. J. Pharm.Innov.2010b, 5, 24-36.
(23). Barrasso D., Ramachandran R. A comparison of model order reduction techniques for a four-dimensional population balance model describing multi-component wet granulation processes. Chem Eng Sci.2012; 80:380–92.
(24). Singh, R., Muzzio, F., Ierapetritou, M., Ramachandran, R. A combined feed-forward/feed-back control system for a QbD based continuous tablet manufacturing process. PROCESSES Journal, 2015, 3, 339-356.
(25). Barros, F. N., Bhaskar, A., Singh, R. A validated model for design and evaluation of control architectures for continuous tablet compaction process. Processes Journal, 2017, 5(4), 76. doi:10.3390/pr5040076
(26). Singh, R., Gernaey, K.V., Gani, R. An ontological knowledge based system for selection of process monitoring and analysis tools. Comput. Chem. Eng.2010, 34(7), 1137-1154.
(27). Haas, N. T., Ierapetritou, M., Singh, R. Advanced model predictive feedforward/feedback control of a tablet press. Journal of Pharmaceutical Innovation, 2017, 1-14, DOI 10.1007/s12247-017-9276-y.
(28). Singh, R., Román-Ospino, A. D., Romañach, R. J., Ierapetritou, M., Ramachandran, R. Real time monitoring of powder blend bulk density for coupled feed-forward/feed-back control of a continuous direct compaction tablet manufacturing process. International Journal of Pharmaceutics, 2015, 495, 612-625. http://dx.doi.org/10.1016/j.ijpharm.2015.09.029
(29). Bhaskar, A., Barros, F. N., Singh, R. Development and implementation of an advanced model predictive control system into continuous pharmaceutical tablet compaction process. International Journal of Pharmaceutics, 2017, 534 (1-2), 159-178.
(30). Singh, R. A novel continuous pharmaceutical manufacturing pilot-plant: Advanced model predictive control. Pharma,2017, Issue 28, PP 58-62. https://www.pharmafocusasia.com/manufacturing/novel-continuouspharmaceutical-manufacturing.