Bacteria was used to treat cancer decades ago. However, this method was mostly replaced by radiotherapy and chemotherapy. In recent years, research on bacterial cancer therapy has revived, and this article introduces the current studies in this field.
Cancer is a deadly disease featured by unchecked cell growth and the potential to spread to different body parts. Despite various therapies, such as surgery, radiation, chemotherapy, targeted therapy, and immunotherapy, cancer remains incurable for many patients, mainly due to treatment resistance and toxic side effects. Thus, complementary or alternative therapies, including bacteria-based cancer therapy, have been explored to overcome these limitations.
Bacterial cancer therapy began decades ago. In 1891, oncologist Dr. Willianm B. Coley (1862-1936) first injected live bacteria into a patient with inoperable cancer, leading to tumourshrinking (Coley 1891; McCarthy 2006). This success led to the development of "Coley's toxins," constituted by Streptococcus pyogenes and Serratia marcescens, which were used to treat sarcomas, lymphomas, myelomas, and melanomas (Hernández-Luna et al., 2018) until the 1960s, with survival rates similar to those of patients diagnosed with cancer in 1983 (Richardson et al., 1999). However, this approach was mostly abandoned later due to the emergence and popularity of radiotherapy and chemotherapy. In recent years, progress in biotechnology, immunology, and molecular biology has led to a revival in bacterial cancer therapy.
Due to the rapid growth of tumour cells, the oxygen level becomes very limited, a condition called hypoxia, in the tumour microenvironment (TME). Severe hypoxia is detected in the TMEs of nearly all solid tumours (Sedighi et
al., 2019; Jing et al., 2019). Thus, many anaerobic bacteria, germs that can survive and grow under an environment lacking oxygen, for example, Salmonella, Clostridium, and Bifidobacterium, have been studied for cancer treatment due to their ability to multiply in the hypoxic tumour tissues preferentially (Sedighi et al., 2019).
Bacteria use two ways to suppress tumour cell growth and progression: stimulating the immune system and directly killing cancer cells. The bacteria- stimulated immune response is the mechanism for bacteria-based cancer immunotherapy (Tang et at., 2022). The immune system contains various types of immune cells, such as lymphocytes (B cells and T cells), natural killer cells, dendritic cells (DCs), macrophages, and neutrophils. When bacteria invade the body, macrophages and neutrophils can attack and kill them. Bacteria that escape the attack by immune cells tend to move to the TME because the hypoxia condition in the TME could suppress the functions of local immune cells (Tang et at., 2022; Huang et al., 2021), which benefits the survival and growth of bacterial and tumour cells. However, bacteria-derived molecules, such as lipopolysaccharide (LPS), peptidoglycan, lipoteichoic acid, and flagella, can stimulate the immune system to kill tumour cells by mechanisms such as binding to pattern recognition receptors (PRRs) from DCs and macrophages and increasing the production of pro-inflammatory cytokines and chemokines in the body (Tang et at., 2022). Live or dead bacteria can be used in bacteria-based cancer immunotherapy. Live bacteria are injected into the body or tumours or taken up into the gut to stimulate the immune response. Dead bacteria or their components are directly injected into the tumours to elicit an increased immune response to attack tumour cells.
Bacteria can directly kill cancer cells by producing bacterial toxins (Huang et al., 2021; Duong et al., 2019; Zahaf et al., 2017), such as diphtheria toxin and pore-forming toxins, or by entering cancer cells to undermine or exploit xenophagy, a type of selective macroautophagy (Uchugonova et al., 2015; Sui et al., 2017). Macroautophagy (autophagy) is a "self-digestion" and stress-responsive process in eukaryotic cells, the type of cells with a nucleus. During autophagy, materials (cargos) inside the cell are wrapped in autophagosomes, a structure with double membranes. An autophagosome fuses with the lysosome, a "suicidal bag" containing digestive enzymes to break down materials, to generate a new structure named autolysosome, where the cargo(s) are broken down into small molecules. Then these small molecules can be used to produce new components and energy to help the cell survive under stress, for example, starvation (Chen et al., 2022). Autophagy is called selective autophagy when it recognises and degrades a specific cargo. Xenophagy is a type of selective autophagy degrading microbes, such as bacteria and viruses.
When bacterial cells enter tumour cells, they can either be killed by xenophagy or survive to cause tumour cell death by undermining or exploiting the xenophagy pathway (Sui et al., 2017) (Figure 1). For example, Salmonella enterica serovar Typhimurium (SeT) (Sui et al., 2017) and Streptococcus pyogenes (Nozawa et al., 2012) can be degraded by xenophagy. In contrast, Mycobacterium tuberculosis and Legionella pneumophila can escape clearance by xenophagy (Kimmey et al., 2016). The detailed mechanisms of xenophagy and its blockage by bacteria are still largely unknown.
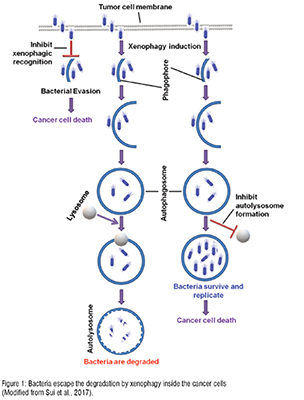
Bacterial cancer therapy can be used as a complementary or alternative therapy to overcome treatment resistance and toxic side effects of radiotherapy, chemotherapy, and targeted therapy. However, it faces challenges (Rommasi 2022):
1. A systemic bacterial infection of the body could render a big risk to a living organism.
2. Low cytotoxicity may require a low dose of bacteria, which might compromise therapeutic efficacy.
3. It is difficult to use bacteria to eradicate all cancer cells in the body, which might lead to cancer recurrence.
Instead of suppressing cancer, bacteria could help cancer cells grow and spread to other parts of the body, a process called metastasis (Galeano Niño et al., 2022). A cancer-suppressing bacterium might mutate to benefit cancer progression.
In recent years, genetically engineered bacteria (GEB) have been generated to express reporter genes, cytotoxic proteins, and tumour-specific antigens (Sedighi et al., 2019; Duong et al., 2019). These bacteria can be modified to have reduced pathogenicity to the host and increased multiplication and cytotoxicity in tumour cells (Low et al., 1999). The use of GEB renders a bright future for bacterial cancer therapy (Gurbatri et al., 2022).
The roles of microbiota in cancer prevention and progression are catching increasing attention from researchers. Gut microbiota can inhibit or promote cancer progression depending on the context (Galeano Niño et al., 2022; Cheng et al., 2020). A recent study reports that the chemotherapeutic drug 5-Fluorouracil (5-FU) inhibits the growth of the cancer-promoting bacterium Fusobacterium nucleatum in the tumours of colorectal cancer (CRC); however, members of the intratumoural microbiota of CRC can modify 5-FU into a nontoxic product that does not inhibit the growth of CRC cells and F. nucleatum cells (LaCourse et al., 2022). This study's findings suggest that intratumoural microbiota's impact on cancer progression and therapy has been undervalued. Intratumoural microbiota likely affects any type of cancer treatment. A maximal therapy efficacy could be reached by using bacterial cancer therapy as a complementary therapy with other cancer treatments.
One promising area of research is using probiotic bacteria in cancer treatment. Probiotic bacteria are considered “good bacteria” to maintain a balanced gut microbiota for helping digestive health and boosting the immune system. They might be safer than other types of bacteria for cancer therapy. Current studies mainly focus on probiotic bacteria's roles in gut microbiota for preventing and treating CRC, breast cancer, and other cancers. Genetic engineering of probiotic bacteria could be an appealing strategy for improving bacterial cancer therapy. For example, the probiotic lactic acid bacterium Pediococcus pentosaceus was genetically engineered to inhibit CRC tumour growth (Chung et al., 2021).
The centuries-old bacterial cancer therapy has been revived in recent years. This treatment depends on two mechanisms to suppress cancer progression, including (1) using live or dead bacteria to stimulate the immune system and (2) facilitating bacteria to directly kill tumour cells via producing bacterial toxins or entering tumour cells to induce cancer cell death. The hurdles to using bacterial cancer therapy include the potential pathogenicity of bacteria to the host and the bacteria's low cytotoxicity in tumour cells. These issues might be overcome by generating GEB. The types of dominant bacteria in intratumoural microbiota can determine whether the microbiota will benefit or antagonise cancer therapy. Gut microbiota is known to affect cancer prevention and treatment. The uptake of probiotic bacteria can improve gut microbiota to help treat cancer, especially CRC. Since probiotic bacteria are generally safe for the body, their genetic engineering is a promising strategy for improving bacterial cancer therapy.
References
1 Coley, W. B. II Contribution to the knowledge of sarcoma. Ann Surg. 1891; 14(3): 199-220.
2 McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissuesarcomas. Iowa Orthop J. 2006; 26: 154-158.
3 Hernández-Luna MA et al. Cancer immunotherapy: Priming the host immune response with live attenuated Salmonella enterica. J Immunol Res. 2018; 2018: 2984247.
4 Richardson MA et al. Coley toxins immunotherapy: A retrospective review. Altern Ther Health Med. 1999; 5(3): 42-7.
5 Sedighi M et al. Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer Med. 2019; 8(6): 3167-3181.
6 Jing X et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019; 18(1): 157.
7 Tang Q et at. Current status and future directions of bacteria-based immunotherapy. Front Immunol. 2022; 13: 911783.
8 Huang X et al. Bacteria-based cancer immunotherapy. Adv Sci (Weinh). 2021; 8(7): 2003572.
9 Duong MT et al. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp Mol Med. 2019; 51(12): 1-15.
10 Zahaf NI et al. Bacterial toxins for cancer therapy. Toxins (Basel). 2017; 9(8): 236.
11 Uchugonova A et al. Imaging the different mechanisms of prostate cancer cell-killing by tumor-targeting Salmonella Typhimurium A1-R. Anticancer Res. 2015; 35(10): 5225-5229.
12 Sui X et al. Bacterial xenophagy and its possible role in cancer: A potential antimicrobial strategy for cancer prevention and treatment. Autophagy. 2017; 13(2): 237-247.
13 Chen Y et al. Editorial: Autophagy-mediated cell survival and death in disease progression and treatment. Front Cell Dev Biol. 2022; 10: 916347.
14 Nozawa T et al. The small GTPases Rab9A and Rab23 function at distinct steps in autophagy during Group A Streptococcus infection. Cell Microbiol. 2012; 14(8): 1149-1165.
15 Kimmey JM et al. Bacterial pathogens versus autophagy: Implications for therapeutic interventions.Trends Mol Med.2016; 22(12): 1060-1076.
16 Rommasi R. Bacterial-based methods for cancer treatment: what we know and where we are. Oncol Ther. 2022; 10(1): 23-54.
17 Galeano Niño JL et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature. 2022; 611: 810-817.
18 Low KB et al. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumortargeting in vivo. Nat Biotechnol. 1999; 17(1): 37‐41.
19 Gurbatri CR et al. Engineering bacteria as interactive cancer therapies. Science. 2022;378(6622): 858-864.
20 Cheng WY et al. The role of gut microbiota in cancer treatment: friend or foe? Gut. 2020;69(10): 1867-1876.
21 LaCourse KD et al. The cancer chemotherapeutic 5-fluorouracil is a potent Fusobacterium nucleatum inhibitor and its activity is modified by intratumoral microbiota. Cell Rep. 2022; 41(7): 111625.
22 Chung Y et al. A synthetic probiotic engineered for colorectal cancer therapy modulates gut microbiota. Microbiome. 2021; 9: 122.